A groundbreaking discovery from biologists at Duke-NUS Medical School and an international research team has revealed a novel mechanism that could transform the treatment of heart disease, particularly ischemic heart disease, a condition caused by restricted blood and oxygen flow to the heart. The team identified a previously unknown protein supercomplex, named SC-XL, which enhances energy production in cells and reduces harmful byproducts, even when their main energy sources fail.
Published in Cell Metabolism, the findings have the potential to revolutionize therapies for disorders linked to deficiencies in cellular energy production, a critical issue in heart disease. This research holds promise for patients with ischemic heart disease, a leading cause of death in Singapore, responsible for nearly 19% of deaths in 2019. The global impact of ischemic heart disease is even more severe, with over 20.5 million deaths annually, a number that is projected to rise significantly due to aging populations and economic disparities.
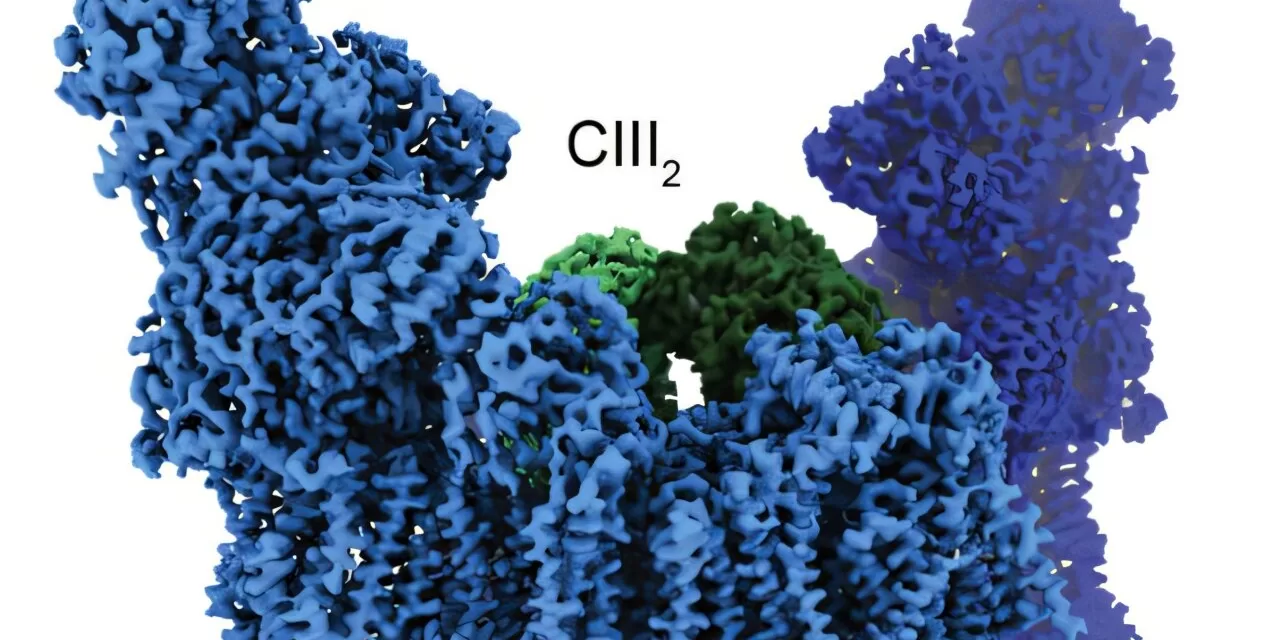
Mitochondria, the powerhouse of cells, produce energy through a process called the electron transport chain, which is essential for heart function. However, in cases of ischemic heart disease, traditional energy production pathways falter due to reduced oxygen flow. This is where the SC-XL supercomplex steps in, helping to enhance energy production and support heart function under stress. SC-XL has been found to improve mitochondrial function even when Complex III, a critical protein complex involved in energy production, is reduced by up to 70%.
Dr. Liang Chao, the study’s first author and a research fellow at Duke-NUS’ Cardiovascular and Metabolic Disorders Program, emphasized the exciting possibilities of these findings, stating, “We’re optimistic about the potential of targeting supercomplexes as a therapeutic strategy for chronic metabolic diseases associated with mitochondrial dysfunction.”
The formation of SC-XL is particularly significant in cases of genetic mutations that impair Complex III, which can lead to the spontaneous creation of SC-XL. This discovery was made possible through advanced high-resolution cryo-electron microscopy, developed in collaboration with Dr. James A. Letts from the University of California-Davis. The team found that SC-XL comprises two subunits each from Complex I and Complex III. This structural formation increases the number of folds in the inner mitochondrial membrane, enhancing the surface area available for energy-producing proteins, thus boosting overall energy production.
Additionally, SC-XL helps improve metabolic health by promoting fatty acid oxidation, which boosts energy production while reducing the harmful accumulation of lipids in cells. The supercomplex also decreases the production of reactive oxygen species (ROS), which are harmful byproducts that can damage cells, further supporting the stability of mitochondrial function.
The study’s lead author, Associate Professor Lena Ho from Duke-NUS, highlighted the importance of these findings in rethinking mitochondrial health. “Although mitochondrial supercomplexes have been studied biochemically for years, we now understand their vital role in maintaining energy production during stress,” she explained. “Our next challenge is to activate these backup systems proactively, rather than waiting for a stress trigger.”
Professor Patrick Tan, senior vice-dean for research at Duke-NUS, added, “This discovery reshapes our understanding of cellular energy systems and opens up new possibilities for treating mitochondrial dysfunction. At Duke-NUS, we’re committed to research that has a real-world impact, and SC-XL supercomplexes represent a promising avenue for improving outcomes for heart disease and metabolic disorders.”
The full study, “Formation of I2+III2 Supercomplex Rescues Respiratory Chain Defects,” was published in Cell Metabolism in 2025.
Disclaimer: The findings of this study are in their early stages, and further research is needed to fully understand the therapeutic potential of SC-XL supercomplexes in human health. The results should not be interpreted as immediate treatment options for heart disease or other related conditions.