Researchers at the University of California San Diego School of Medicine have published new findings that could fundamentally change the way we approach anti-aging efforts. Their study, published in Nature Aging, describes a link between the two most accepted explanations for aging: random genetic mutations and predictable epigenetic modifications.
The epigenetic clock theory, widely used as a measure of biological aging, suggests that aging occurs due to the accumulation of epigenetic modifications, minor changes to the chemical structure of DNA that do not alter the underlying sequence, but instead change which genes are on or off. Unlike mutations, epigenetic modifications can also be reversed in some cases.
“Major research institutions and companies are betting on turning back the epigenetic clock as a strategy to reverse the effects of aging, but our research suggests that this may only be treating a symptom of aging, not the underlying cause,” said co-corresponding author Trey Ideker, Ph.D., a professor at UC San Diego School of Medicine and UC San Diego Jacobs School of Engineering.
The new research suggests that mutations are responsible for the observed epigenetic changes. This could fundamentally change the way we approach anti-aging efforts in the future.
Somatic Mutation Theory vs. Epigenetic Clock Theory
There are two prevailing theories about the relationship between aging and DNA.
- The somatic mutation theory suggests that aging is caused by the accumulation of mutations, permanent changes in our DNA sequence that occur randomly.
- The epigenetic clock theory suggests that aging occurs due to the accumulation of epigenetic modifications.
New Study Links Mutations to Epigenetic Changes
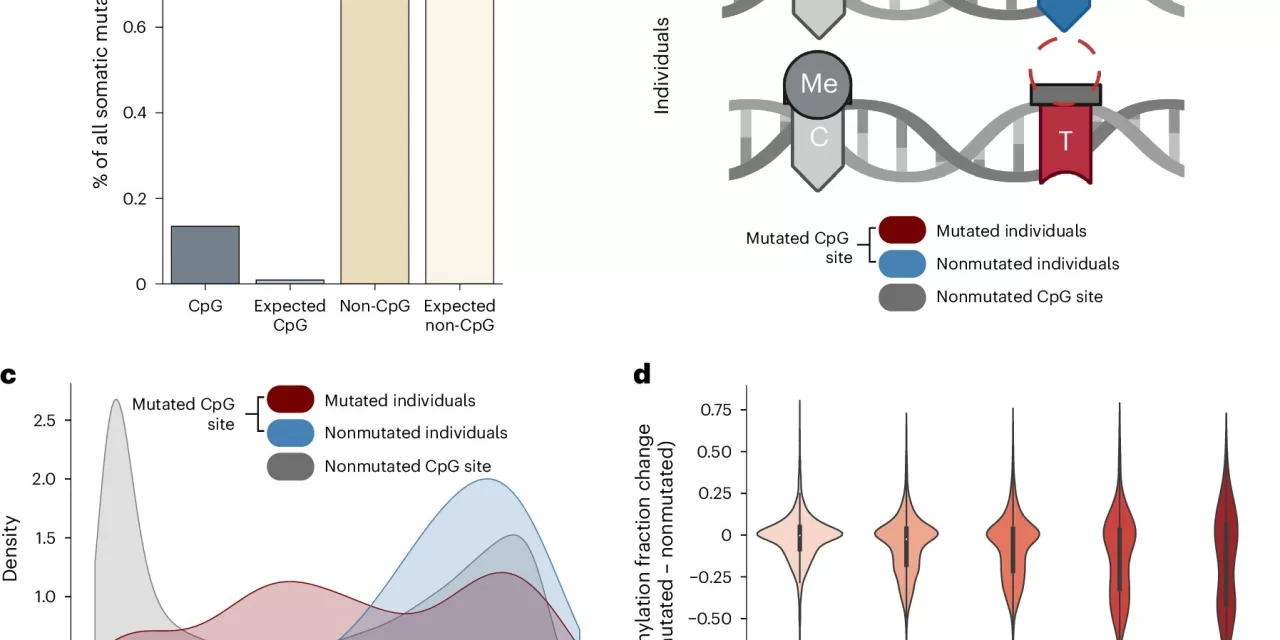
To answer the question of why epigenetic clocks tick, researchers analyzed data from thousands of patients. By comparing genetic mutations to epigenetic modifications, they found that mutations were predictably correlated with changes in DNA methylation, one type of epigenetic modification.
They found that a single mutation could cause a cascade of epigenetic changes across the genome, not just where the mutation occurred. Using this relationship, the researchers were able to make similar predictions of age using either mutations or epigenetic changes.
“Our study demonstrates for the first time that epigenetic changes are intricately and predictably tied to random genetic mutations,” said first author Zane Koch, a Ph.D. candidate in bioinformatics at UC San Diego.
Implications for Anti-Aging Therapies
The study’s findings have important implications for the development of new therapies aimed at preventing or reversing aging.
“If somatic mutations are the fundamental driver of aging and epigenetic changes simply track this process, it’s going to be a lot harder to reverse aging than we previously thought,” added co-corresponding author Steven Cummings, M.D., executive director of the San Francisco Coordinating Center at UC San Francisco and senior research scientist at Sutter Health’s California Pacific Medical Center Research Institute.
“This shifts our focus from viewing aging as a programmed process to one that’s largely influenced by random, cumulative changes over time.”
The study’s authors note that further research is needed to fully understand the relationship between somatic mutations and epigenetic changes in aging. However, the study’s findings provide a major breakthrough in our understanding of the aging process.