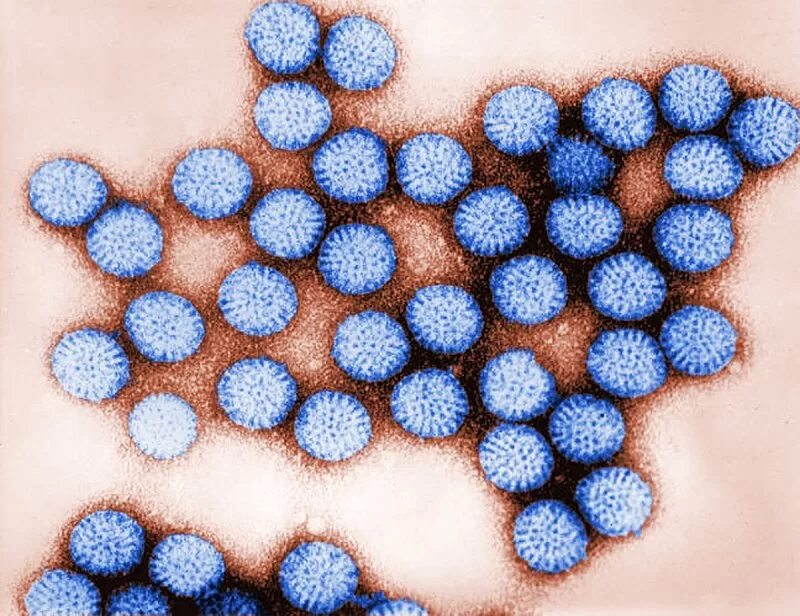
January 19, 2025 – Researchers at Baylor College of Medicine and partner institutions have uncovered critical new insights into how rotavirus, a leading cause of severe gastroenteritis in children, manipulates cellular processes to increase disease severity. Published in Science Advances, the study is among the first to demonstrate that the rotavirus protein NSP4 plays a crucial role in manipulating calcium signaling both within infected cells and in surrounding uninfected cells. These disruptions in calcium signaling have far-reaching effects, amplifying the severity of the infection and providing potential new targets for preventing and treating rotavirus infections.
Rotavirus is responsible for one-quarter of severe cases of pediatric acute gastroenteritis, a condition marked by watery diarrhea, vomiting, fever, and abdominal pain. Despite advancements like oral rehydration therapy and live-attenuated vaccines, nearly 500,000 children worldwide continue to die from this condition each year. This new study shines light on NSP4, a viral protein essential to the virus’s ability to trigger these distressing symptoms.
Dr. Joseph Hyser, corresponding author of the study and Associate Professor of Molecular Virology at Baylor College of Medicine, explained, “Rotavirus infection is still a major global health issue, and there is significant room for improvement in our current strategies to combat it. Our research reveals that NSP4 is a key player in how rotavirus disrupts calcium signaling, and this discovery could be pivotal in developing better treatments.”
The research team’s findings stem from their previous observation that rotavirus infection induces abnormal calcium signals, known as “intercellular calcium waves,” which travel from infected cells to neighboring uninfected cells. Inhibiting these waves was found to reduce the severity of the disease. However, how the virus triggers this calcium signaling remained unclear.
Through advanced experimental models including human and porcine strains of rotavirus, as well as genetically modified strains, the researchers discovered that NSP4 was both necessary and sufficient to initiate calcium waves. Remarkably, the expression of NSP4 alone in cells—without rotavirus infection—was enough to induce calcium signaling similar to that seen during infection.
Moreover, the study revealed that NSP4 from attenuated rotavirus strains, which cause milder symptoms, induced fewer calcium waves compared to NSP4 from more virulent strains. By introducing the attenuated NSP4 into a virulent strain, the researchers observed a marked reduction in calcium wave production and a decrease in the severity of diarrhea in animal models.
“This study highlights the importance of NSP4 in generating calcium waves, a key factor in rotavirus’s ability to cause disease,” said Hyser. “It is clear that the ability to produce these waves is tightly linked to NSP4’s role in disease severity, immune response activation, and the overall impact of the virus.”
The team also found that calcium waves triggered by NSP4 not only contributed to the severity of rotavirus infection but also played a role in the immune system’s recognition of the virus. This discovery may extend to other viruses with similar proteins that manipulate calcium signaling, opening the door for broader applications of this research.
The study was conducted in collaboration with researchers from Indiana University and Stanford University School of Medicine. The work was led by J. Thomas Gebert, Francesca J. Scribano, Kristen A. Engevik, and several others, who all contributed to understanding the complex role of NSP4 in rotavirus infection.
These findings could pave the way for new therapeutic strategies targeting NSP4 to reduce the impact of rotavirus and similar viral infections.
For more information, visit the study published in Science Advances here.