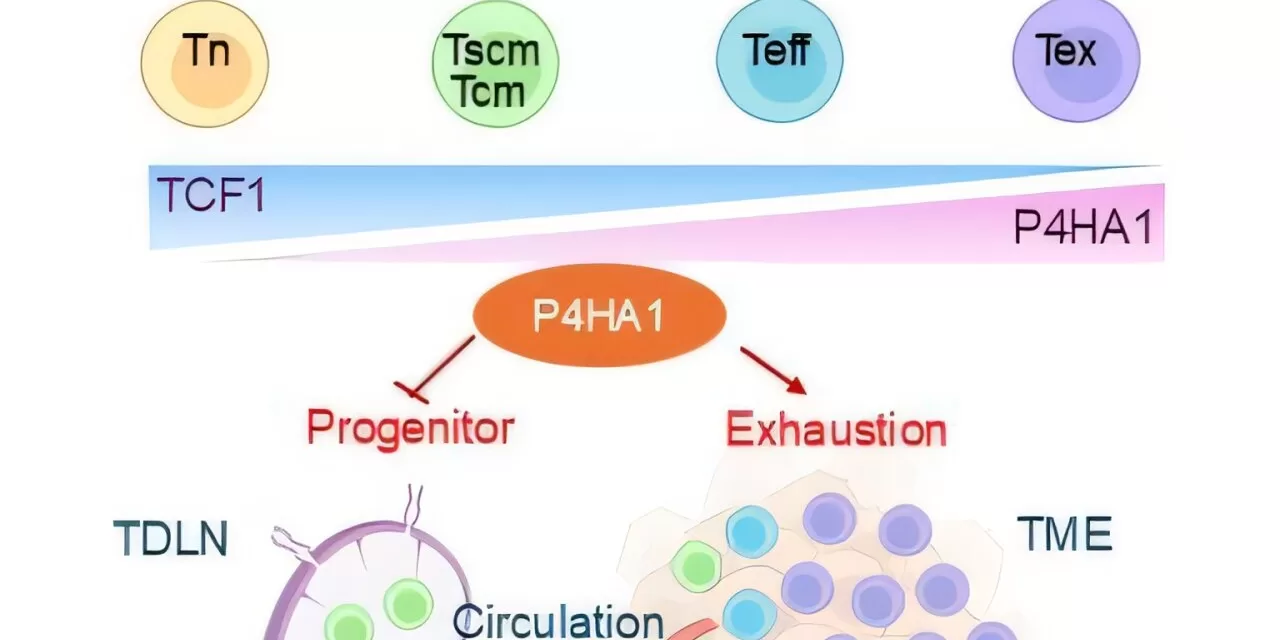
Singapore – December 2024 – In a groundbreaking discovery, scientists from ASTAR Genome Institute of Singapore (ASTAR GIS) have identified a key enzyme, P4HA1 prolyl hydroxylase, as a promising target for cancer immunotherapy. This enzyme, which is strongly induced in CD8+ T cells within solid cancers, plays a crucial role in weakening immune responses, making it a key factor in the difficulty of treating solid tumors.
CD8+ T cells are the immune system’s primary defenders against cancer, but P4HA1 disrupts their energy production, diminishing their ability to fight cancer and form lasting anti-cancer immunity. This discovery, published in Cancer Cell in December 2024, suggests that inhibiting P4HA1 could restore the function of immune cells, improve immune memory, and aid in tumor shrinkage, offering a potential solution to combat immune-resistant tumors.
Through their research, scientists demonstrated that small molecule compounds targeting P4HA1 could not only revive immune cell function but also sustain long-term immunity, preventing tumor relapse. In mouse models of immune-resistant tumors, this inhibition showed significant efficacy in reducing tumor size and improving immune responses.
Moreover, P4HA1’s potential extends beyond natural immune responses. The researchers also found that targeting this enzyme could enhance the effectiveness of CAR-T cell therapy, a specialized treatment that involves modifying a patient’s immune cells to better fight cancer. By targeting P4HA1, researchers were able to make these modified immune cells stronger and more potent.
Additionally, P4HA1 levels were shown to increase in blood immune cells as cancer progressed and relapsed, correlating with a patient’s response to immunotherapies. This positions P4HA1 as not only a therapeutic target but also as a potential biomarker for monitoring cancer progression and treatment outcomes.
One of the key challenges in cancer immunotherapy has been targeting metabolic and epigenetic regulators that control T cell function. While many of these regulators are vital to normal T cell activity, they are difficult to target without affecting healthy cells. P4HA1, however, is expressed at low levels in naïve and memory T cells but becomes significantly upregulated in exhausted T cells within the tumor microenvironment, making it an ideal target for therapies aimed at activated or exhausted T cells. This specificity offers a promising opportunity to improve both cancer treatment and monitoring.
Professor Yu Qiang, Senior Group Leader of the Laboratory of Precision Cancer Medicine at A*STAR GIS and lead author of the study, emphasized the significance of the research, stating, “Our work bridges fundamental science and clinical applications. Based on this discovery, we are committed to developing optimized P4HA1-targeting strategies, including chemical inhibitors and next-generation CAR-T cell platforms, to deliver efficient and feasible treatment solutions for solid tumors.”
Dr. Wan Yue, Executive Director of A*STAR GIS, also highlighted the clinical implications, saying, “The findings on P4HA1’s potential as a biomarker for cancer progression and immunotherapy resistance could enable more precise monitoring and personalized treatment strategies in the future, improving immunotherapy outcomes and advancing patient care.”
The study points to an exciting new frontier in cancer immunotherapy, where targeting P4HA1 could enhance both natural immune responses and advanced therapies, offering hope for more effective and long-lasting treatments for solid tumors.
For more information, refer to the original research: Shijun Ma et al, Targeting P4HA1 promotes CD8+ T cell progenitor expansion toward immune memory and systemic anti-tumor immunity, Cancer Cell (2024). DOI: 10.1016/j.ccell.2024.12.001.