In the wake of the COVID-19 pandemic, the world witnessed the urgent need for more efficient and cost-effective viral testing systems. While the rapid development of vaccines and public awareness campaigns significantly reduced the transmission of COVID-19, experts are now predicting future threats of co-infections, or “twindemics,” where multiple viruses strike at the same time. This raises concerns about the possibility of further viral outbreaks that could place additional strain on healthcare systems worldwide.
To combat these potential risks, a team of scientists from the Republic of Korea has introduced an innovative solution—a diagnostic system that could transform how we detect viral infections. Led by Professor Eunjung Kim from Incheon National University (INU), the team developed the TwinDemic Detection (TDD) system, designed for the simultaneous detection of two viral pathogens: SARS-CoV-2 (the virus responsible for COVID-19) and the influenza A virus (IAV). This breakthrough technology was detailed in their recent publication in the journal Sensors and Actuators B: Chemical.
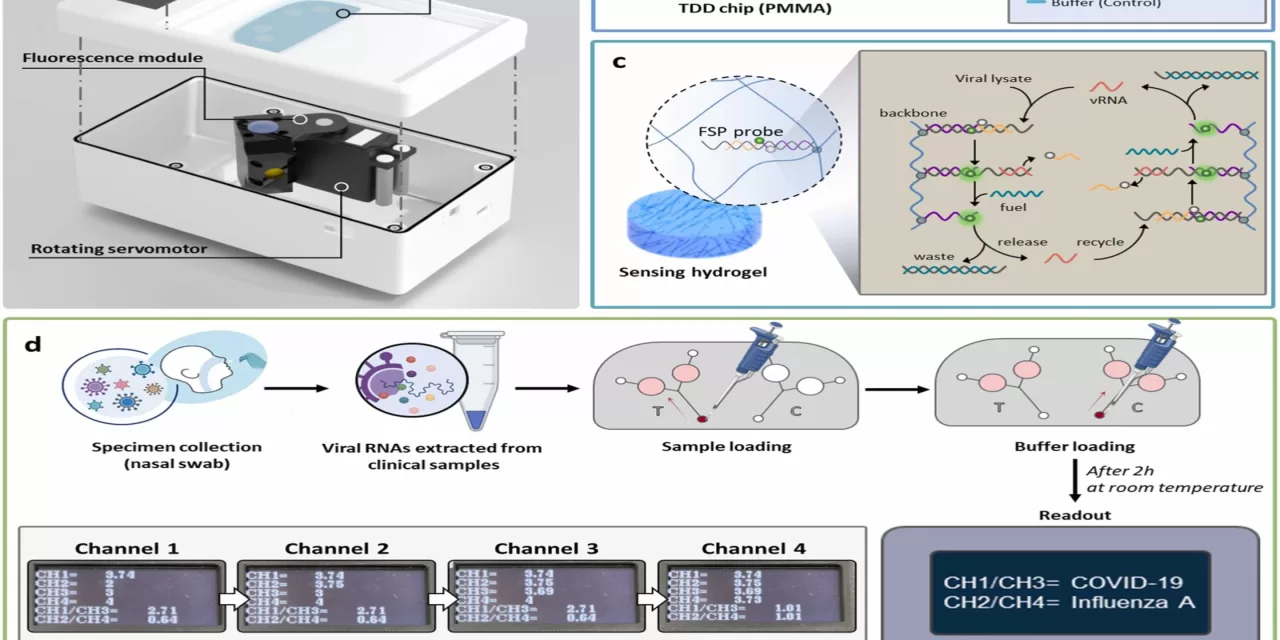
The TDD system utilizes a transparent poly(methyl methacrylate) microfluidic chip embedded with hydrogel-based, enzyme-free gene detection sensors. These sensors are custom-designed to target the two viruses, amplifying their fluorescence signals when they bind to the viral DNA. In addition, the TDD system is paired with a handheld fluorescence reader, making it portable and user-friendly for on-site, point-of-care testing.
One of the key advantages of this diagnostic tool is its cost-effectiveness and ease of use. Unlike traditional methods such as Reverse Transcriptase-quantitative PCRs (RT-qPCR), which require expensive equipment and reagents, the TDD system offers a more accessible alternative for regions with limited resources. The detection limit for SARS-CoV-2 is 0.46 picomolar (pM), and for IAV, it’s 0.39 pM, making the TDD highly sensitive to even low levels of viral presence.
To test the accuracy of the system, the research team analyzed 15 nasopharyngeal swabs from healthy individuals, COVID-19 patients, and patients with influenza A. The results were promising: for SARS-CoV-2, the TDD system correctly identified positive cases 93.3% of the time, and negative samples were accurately predicted 96.7% of the time. For IAV, the system showed a perfect 100% accuracy in identifying positive samples and 96.7% accuracy for negative samples.
“The application of our TDD system can be further expanded by introducing additional channels and sensing hydrogels on the microfluidic chip, as well as integrating highly sensitive nucleic acid amplification systems,” explained Prof. Kim. “This would allow for the detection and differentiation of a broader range of viruses, strengthening our ability to respond to future health threats.”
The TDD system’s ability to rapidly and accurately detect multiple respiratory viruses in a single test could be a game-changer for healthcare providers, allowing them to make timely and informed decisions regarding patient treatment. With the ongoing challenges posed by viral infections, this new tool has the potential to significantly enhance global health responses, especially in regions facing resource limitations.
As the world braces for the possibility of future viral outbreaks, innovations like the TDD system provide hope for improved diagnostic capabilities, helping to ensure faster and more effective responses in the face of evolving health threats.
For more information, refer to Jaewoo Lim et al.’s study titled TwinDemic Detection: A Non-Enzymatic Signal Amplification System for On-Site Detection of Multiple Respiratory Viruses, published in Sensors and Actuators B: Chemical (2024). DOI: 10.1016/j.snb.2024.136933.