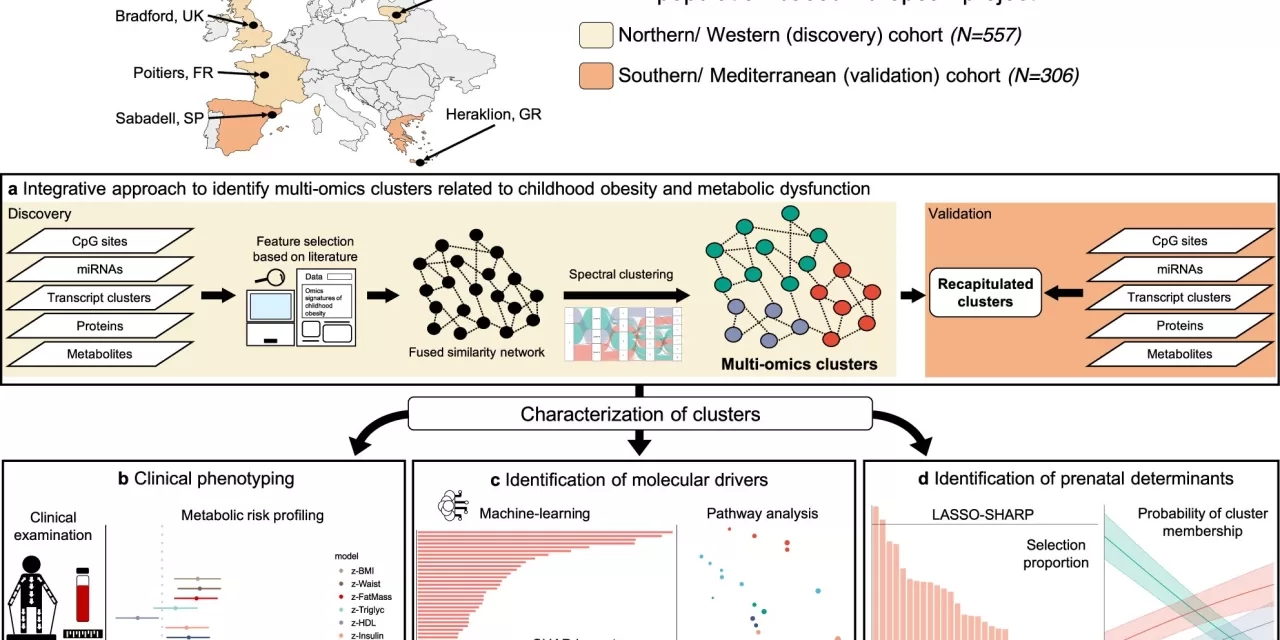
A groundbreaking molecular study led by the Barcelona Institute for Global Health (ISGlobal) has uncovered crucial insights into the biological pathways linked to childhood obesity and metabolic dysfunction. The research, which examined over 800 children across Europe, utilized a pioneering “multi-layered omics” approach to provide a comprehensive understanding of how obesity develops and how it is influenced by environmental risk factors from prenatal life.
Childhood obesity remains a significant public health challenge, with one in ten children affected across Europe. This condition predisposes them to long-term health problems, including metabolic disorders and cardiovascular disease. However, despite its widespread nature, the biological mechanisms underlying obesity-related health issues are not fully understood, particularly why not all children with obesity develop metabolic complications.
To fill this knowledge gap, the ISGlobal team employed advanced techniques to analyze gene expression, proteins, and metabolites in blood samples. By examining these biological markers alongside detailed health data and prenatal environmental information, researchers were able to uncover distinct clusters within the children, providing a clearer picture of the factors contributing to obesity.
Understanding the Biology Behind Obesity
The study revealed three distinct clusters among the children, one of which showed significantly higher body fat and clear signs of metabolic complications, such as inflammation markers. These inflammation markers, which point to an overactive immune system, are believed to play a key role in triggering insulin resistance and chronic inflammation—a known pathway to metabolic dysfunction.
“By focusing on clusters derived from multi-omics profiles, our approach offers a deeper understanding of the biological mechanisms involved in metabolic health, beyond what traditional clinical markers can show,” explained first author Nikos Stratakis.
Prenatal Environmental Risk Factors
The research also pinpointed the importance of prenatal exposures in shaping a child’s risk for obesity and metabolic problems. Specifically, the study found that the mother’s weight before pregnancy significantly impacted whether a child fell into the high-risk group for obesity-related complications.
Environmental exposures during pregnancy also played a critical role, with variations observed between regions in Europe. In Northern and Western Europe, maternal exposure to perfluorooctanoate (PFOA)—an industrial chemical found in non-stick coatings—was identified as a risk factor. Meanwhile, in Southern and Mediterranean Europe, maternal mercury exposure, likely from higher fish consumption, was found to be a significant contributor.
A Path Forward for Prevention
These findings highlight the potential for early intervention and the importance of targeting modifiable risk factors during prenatal life. The researchers suggest that personalized prevention strategies, taking regional and environmental differences into account, could be key in tackling the childhood obesity crisis.
“These results emphasize the need to tailor prevention guidelines to different country contexts and populations,” said Martine Vrijheid, ISGlobal researcher and senior author of the study. “Identifying modifiable risk factors early in life is crucial for curbing the long-term health impacts of childhood obesity.”
This study, part of the Human Early Life Exposome (HELIX) project, followed children from various regions in Europe, including Northern Europe (Bradford, UK; Poitiers, France) and Southern Europe (Sabadell, Spain; Heraklion, Greece), and was published in Nature Communications.
The research offers a promising new approach to understanding the complex biology of childhood obesity and may pave the way for more effective prevention strategies in the future.
For more information, see the full study: Nikos Stratakis et al, Multi-omics architecture of childhood obesity and metabolic dysfunction uncovers biological pathways and prenatal determinants, Nature Communications (2025). DOI: 10.1038/s41467-025-56013-7