New Research from Rutgers Health Offers Hope to EPP Patients
A common antihistamine, chlorcyclizine, could become a groundbreaking treatment for erythropoietic protoporphyria (EPP), a rare genetic disorder that causes severe liver damage and extreme sensitivity to sunlight. According to a study published in Cellular and Molecular Gastroenterology and Hepatology, researchers from Rutgers Health have discovered that this decades-old allergy drug could potentially prevent liver damage and reduce the need for liver transplants in EPP patients.
EPP affects about 4,000 people in the United States, with a small percentage developing liver complications severe enough to require transplantation. Currently, liver transplant is the only treatment option for patients facing severe liver damage, but the procedure is costly and dependent on donor organ availability.
“There is an unmet need for these patients,” said Dr. Bishr Omary, senior vice chancellor for academic affairs and research at Rutgers Health and senior author of the study. “Liver transplantation, while life-saving, is not always an option, especially with the limited availability of donor organs.”
EPP causes the accumulation of toxic levels of protoporphyrin in the liver, bone marrow, red blood cells, and plasma, leading to potential liver damage and skin issues. Despite its rarity, there is little commercial incentive for pharmaceutical companies to develop treatments for EPP, which led Omary’s team to test existing medications for their efficacy.
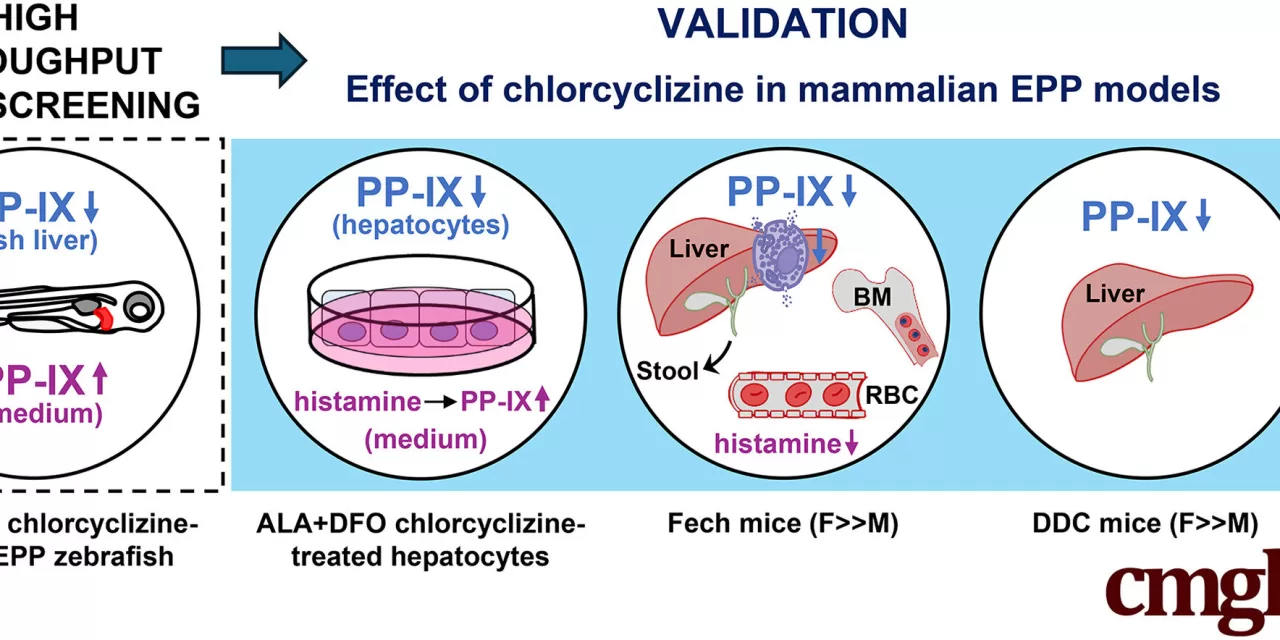
In their study, researchers screened more than 2,500 compounds, including many FDA-approved drugs, using a zebrafish larvae model to observe the buildup of toxic compounds. The transparent larvae allowed the team to visually track the fluorescent porphyrin buildup and easily assess the effectiveness of each compound.
“We could directly observe the results under the microscope,” explained Ning Kuo, the study’s first author. “If I saw glowing porphyrin, it meant the treatment didn’t work. If I didn’t, it showed the liver was successfully clearing the toxin.”
One of the key findings was that chlorcyclizine, an over-the-counter antihistamine, reduced hepatic protoporphyrin accumulation in female mice, decreasing liver injury and the levels of protoporphyrin in bone marrow and red blood cells. This effect was also accompanied by increased excretion of the toxic compound in stool. Interestingly, this effect was sex-specific, with female mice showing better results than male mice, likely due to the different rates of drug metabolism between genders.
In further experiments with EPP liver cell cultures, researchers showed that the histamine pathway plays a role in promoting porphyrin accumulation, a process blocked by antihistamines like chlorcyclizine. The study also revealed that chlorcyclizine worked through multiple mechanisms, including helping the liver clear toxic buildup and reducing inflammation.
These findings offer potential for a more accessible treatment option for EPP patients, potentially preventing liver damage much earlier in the disease process. Chlorcyclizine, already known for its safety due to decades of use in allergy treatment, could fast-track the development of clinical trials for its use in EPP.
The research team is now focused on securing support for a clinical trial to evaluate chlorcyclizine’s effectiveness in treating both liver and skin manifestations of EPP. Additionally, a Phase 2 clinical trial is already underway to test cimetidine, an antacid, for treating the skin aspects of the disease. There is also hope that different antihistamines may work together synergistically for enhanced treatment.
“Given their well-established safety profile, we’re hopeful we can fast-track the clinical trials for chlorcyclizine, either alone or in combination with cimetidine,” said Kuo.
The findings could be a breakthrough for EPP patients, offering a simpler and more accessible treatment option, potentially reducing reliance on organ transplants and improving quality of life for those affected by this rare condition.
Source: Cellular and Molecular Gastroenterology and Hepatology (2025). DOI: 10.1016/j.jcmgh.2025.101463