In a groundbreaking study, a team of researchers from Concordia University’s Departments of Biology and Physics has proposed an innovative method to enhance the immune system’s response to cancer tumors. Their research, published in the journal Frontiers in Immunology, introduces a novel use of ultrasound-guided microbubbles—a technology commonly used in medical imaging and drug delivery—to activate immune cells and improve their cancer-fighting capabilities.
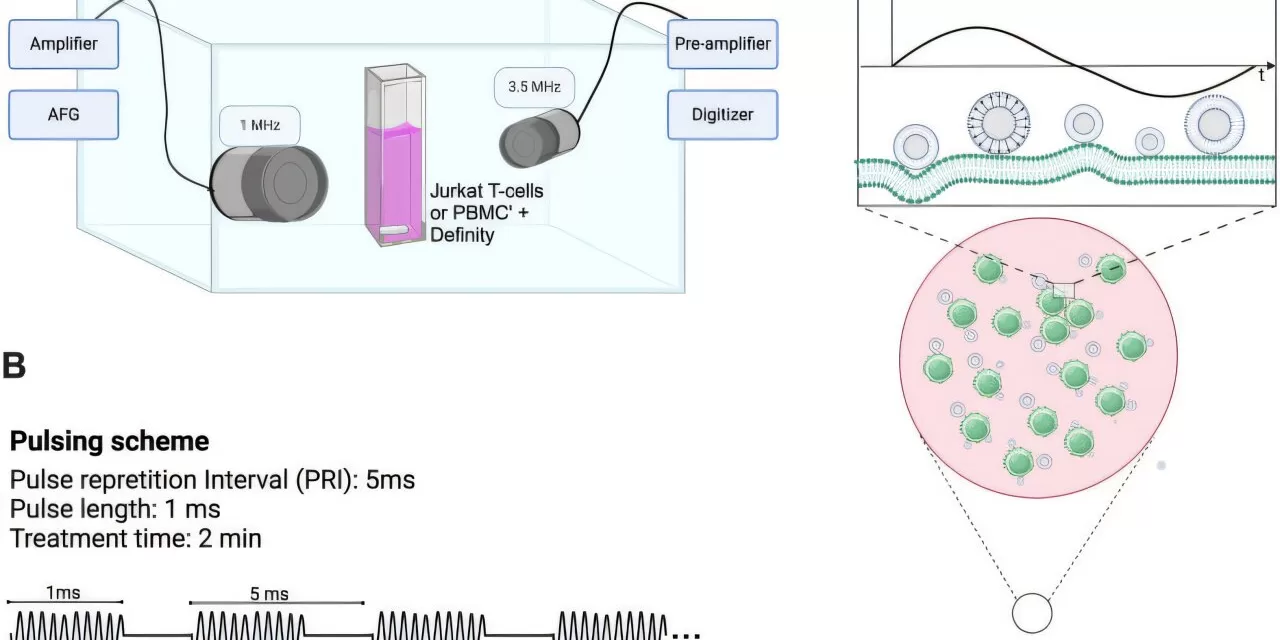
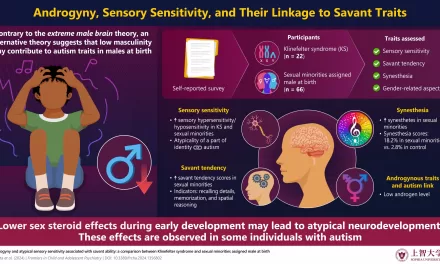
The study focuses on a process that utilizes ultrasound to modify the behavior of T cells, which play a crucial role in the body’s immune defense. By using tightly focused ultrasound beams combined with clinically approved contrast agent microbubbles, the researchers were able to increase the permeability of T cell membranes. This alteration allows T cells to secrete cytokines—signaling molecules that are critical for activating immune responses—into the tumor microenvironment.
These microbubbles, when hit with ultrasound, vibrate at a high frequency, creating a push-pull effect on the T cell membranes. This vibration mimics the natural immune response triggered by the presence of an antigen, prompting the T cells to release cytokines that would otherwise be suppressed by the tumor’s hostile environment. Importantly, this technique does not harm the T cells themselves, providing a non-invasive method to boost immune activity.
“This approach is part of the emerging field of cancer immunotherapy, where we harness the body’s own immune cells to fight cancer,” said Brandon Helfield, an associate professor of biology and physics and the study’s supervising author. “By combining ultrasound and microbubbles, we aim to modulate brain immunology and enhance immune responses in a targeted manner.”
One of the major challenges in cancer immunotherapy is overcoming the tumor’s ability to deactivate T cells once they enter the tumor. The researchers believe that microbubbles can help reactivate these suppressed immune cells. “This process will allow T cells to release vital proteins that are needed to stimulate immune and blood cells, creating a positive feedback loop,” explained Ph.D. candidate Ana Baez, the lead author of the paper.
The study found that ultrasound-induced changes to cytokine secretion were time-dependent. Over 48 hours, the secretion of cytokines increased by up to 3.6 times compared to untreated cells. However, the amount of cytokines released generally decreased when ultrasound made the T cell membranes more permeable.
Though the results are based on preliminary benchtop experiments, the researchers are optimistic that their findings could lead to improved cancer therapies. “We already use microbubbles clinically for imaging purposes,” Helfield said. “In the future, we could use them for both imaging and therapy, precisely targeting the T cells where they are most needed.”
Additionally, the researchers hope to combine this technique with cancer-fighting drugs in the future, further enhancing its therapeutic potential. The non-invasive nature of this method also offers the possibility of repeated treatments, which could improve patient outcomes over time.
This research was a collaborative effort involving Davindra Singh, Stephanie He, Mehri Hajiaghayi, Fatemeh Gholizadeh, and Peter Darlington.
For further details, see the full study: Baez, A., et al. (2024). Immunomodulation of human T cells by microbubble-mediated focused ultrasound, Frontiers in Immunology. DOI: 10.3389/fimmu.2024.1486744.