A groundbreaking study led by researchers at Stanford Medicine has uncovered a way to predict how long immunity from vaccines will last. By analyzing blood samples from vaccinated individuals, scientists identified a molecular signature that could forecast the durability of immune responses. The study highlights the role of a surprising blood cell, the megakaryocyte, typically associated with blood clotting, in influencing vaccine immunity.
For years, scientists have grappled with why some vaccines provide long-lasting immunity, while others lose their effectiveness quickly. For example, while the measles, mumps, and rubella (MMR) vaccine offers lifetime protection after just two doses, the flu vaccine’s effectiveness fades rapidly after a few months. This new study sheds light on the underlying mechanisms behind this variation.
Bali Pulendran, Ph.D., a professor of microbiology and immunology at Stanford, explained, “The question of why some vaccines induce durable immunity while others do not has been one of the great mysteries in vaccine science. Our study defines a molecular signature in the blood, induced within a few days of vaccination, that predicts the durability of vaccine responses and provides insights into the fundamental mechanisms underlying vaccine durability.”
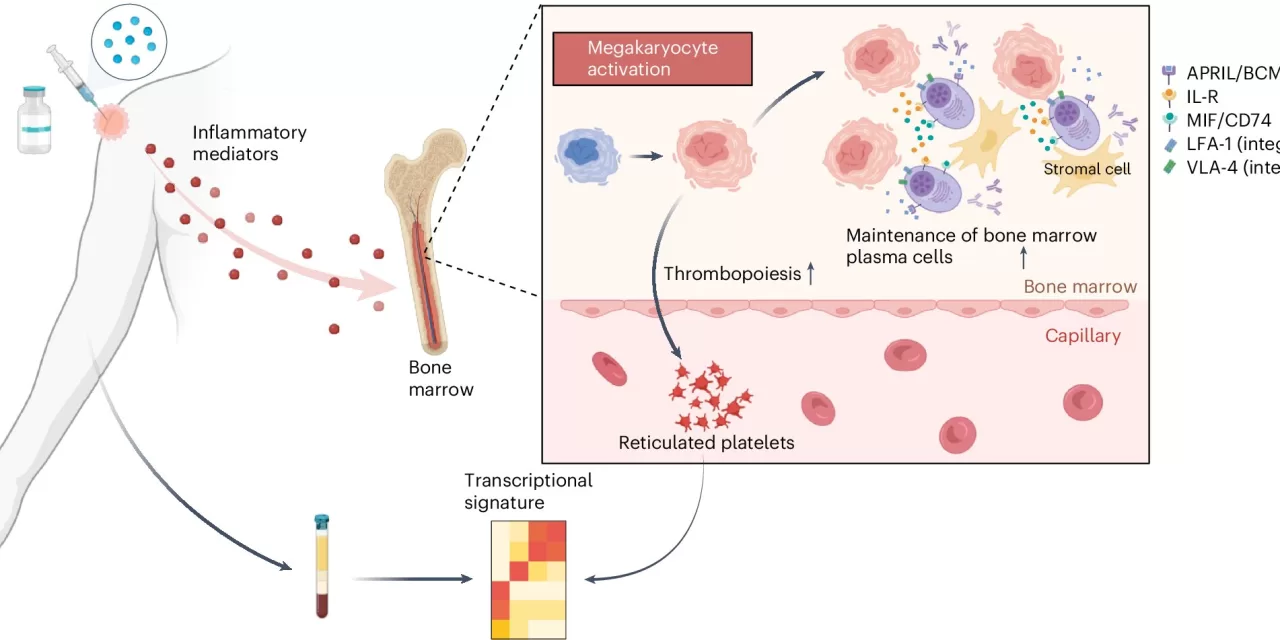
The study was published today in Nature Immunology. The research team, led by Pulendran, followed 50 healthy volunteers who received an experimental H5N1 bird flu vaccine, either with or without an adjuvant—a substance that enhances the immune response. By analyzing blood samples at multiple time points following vaccination, the researchers found a molecular signature associated with longer-lasting antibody responses. This signature was primarily reflected in small bits of RNA found within platelets, which are cells that help form blood clots.
Interestingly, platelets are derived from megakaryocytes in the bone marrow, and these platelets carry small pieces of RNA from megakaryocytes as they circulate in the bloodstream. Although megakaryocytes are difficult to study directly, the RNA within platelets provides a proxy for the cells’ activity.
“Platelets are a bellwether for what is happening with megakaryocytes in the bone marrow,” Pulendran said.
Further experiments showed that increasing megakaryocyte activity in mice led to a sixfold increase in antibody levels months after vaccination. The team found that activated megakaryocytes produce molecules that support the survival of plasma cells, the cells responsible for producing antibodies. When these molecules were blocked, the survival of plasma cells was reduced.
The researchers also found that the same molecular signature in platelets was associated with longer-lasting antibody production across a range of vaccines, including those for influenza, yellow fever, malaria, and COVID-19.
“By identifying these molecular signatures, we can now predict which vaccines will provide longer-lasting protection and which individuals may require a booster,” said Pulendran.
Looking forward, the research team aims to investigate why some vaccines trigger stronger megakaryocyte activation than others, which could lead to the development of more effective vaccines. Additionally, they plan to create a simple PCR test—referred to as a “vaccine chip”—to assess the durability of vaccine responses just days after vaccination. This test could expedite vaccine clinical trials and allow for more personalized vaccine schedules.
As vaccine research progresses, understanding the role of megakaryocytes could provide a key piece of the puzzle in improving vaccine durability and tailoring immunization strategies to individual needs.
For more information, see the study System vaccinology analysis of predictors and mechanisms of antibody response durability to multiple vaccines in humans published in Nature Immunology (2025). DOI: 10.1038/s41590-024-02036-z.