A groundbreaking study led by Yale researchers has identified a potential molecular mechanism behind lissencephaly, a rare and severe brain malformation disorder. This disorder, often associated with seizures and intellectual disability, has no known cure. However, the new research, published in Nature on January 1, 2025, suggests a promising treatment approach, offering hope for those affected by these conditions.
Lissencephaly, which translates to “smooth brain,” is a spectrum of genetic disorders in which the brain fails to develop its usual folds, leading to significant neurological impairments. The condition results from mutations in genes that are critical for brain development. Researchers have been investigating the genetic causes and potential treatments for years, but until now, no effective treatment has emerged.
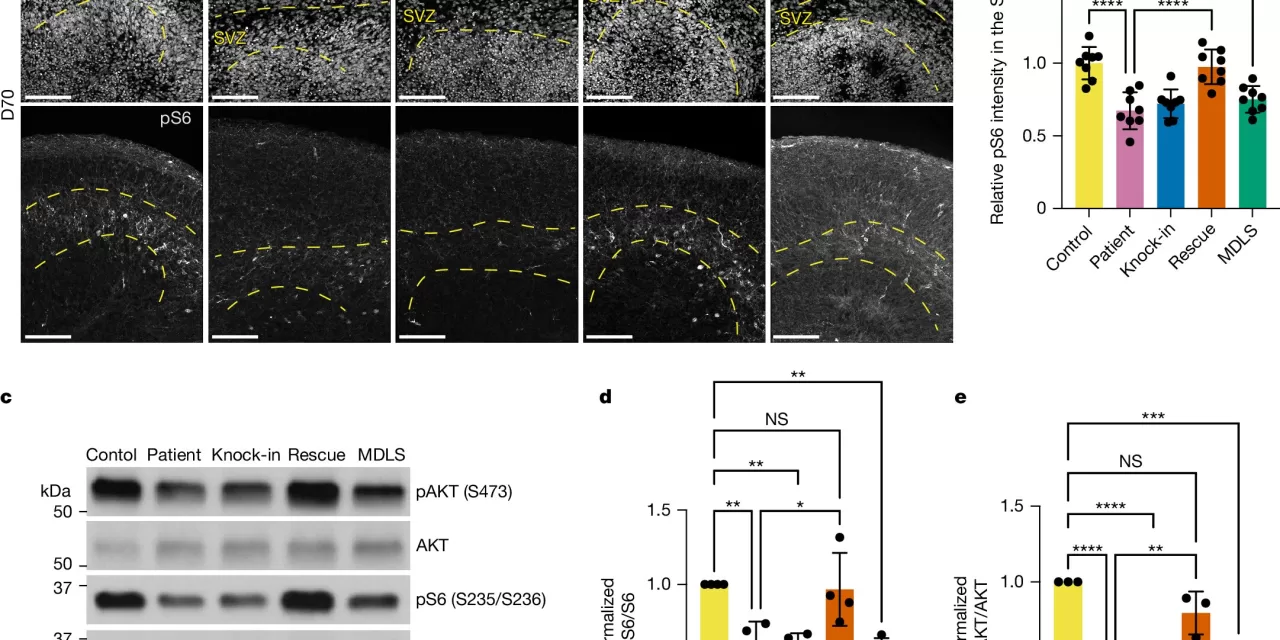
The Yale study reveals a new understanding of the molecular underpinnings of some forms of lissencephaly and introduces a drug that may prevent and even reverse the brain malformations associated with the disorder. The research team, including Angeliki Louvi, Professor of Neurosurgery and Neuroscience at Yale School of Medicine (YSM), and Murat Gunel, Sterling Professor of Neurosurgery at YSM, discovered that the mTOR (mammalian target of rapamycin) pathway, which regulates various aspects of cellular metabolism, is disrupted in lissencephaly.
“While previous studies have linked several genes to lissencephaly, the exact molecular cause remained unclear,” Louvi explained. “Our research identifies an underperforming mTOR pathway as a key factor contributing to these brain malformations.”
The study builds on years of research conducted by the Yale Program in Neurogenetics, which has been collecting genetic data from patients with brain malformations. Kaya Bilguvar, Associate Professor at YSM, who is also a co-senior author of the study, highlighted the long-term commitment of families and patients who have contributed to this important work over the years.
In their study, the researchers used patients’ skin cells, reprogramming them into neurons that developed into brain organoids—small, three-dimensional replicas of developing brains. These organoids showed similar malformations to those seen in lissencephaly, including a thicker-than-usual cortex and a lack of brain folds. After identifying the dysregulation in the mTOR pathway, the researchers tested a drug that activates mTOR signaling. Remarkably, they found that the drug could reverse the structural brain abnormalities in the organoids, depending on the timing of the treatment.
“This could have significant implications for future treatments,” said Ce Zhang, the study’s lead author. “Currently, there is no way to slow or reverse the brain malformations in lissencephaly. Our findings suggest that mTOR activators might provide a potential treatment for patients across the lissencephaly spectrum.”
The researchers are now focused on determining whether mTOR dysregulation is a factor in other genetic forms of lissencephaly and how this underactivity leads to the disorder. They also aim to explore clinical applications for mTOR activators in patients with these conditions.
“This study underscores the delicate balance required for healthy brain development,” Louvi noted. “We hope our findings will pave the way for treatments that address the root cause of lissencephaly, rather than just managing its symptoms.”
With further research and clinical trials, mTOR activators could offer new hope for patients with lissencephaly, ultimately providing a treatment option for a condition that has, until now, been considered untreatable.
For more information on the study, refer to: Ce Zhang et al, Dysregulation of mTOR signalling is a converging mechanism in lissencephaly, Nature (2025). DOI: 10.1038/s41586-024-08341-9.