January 2, 2025
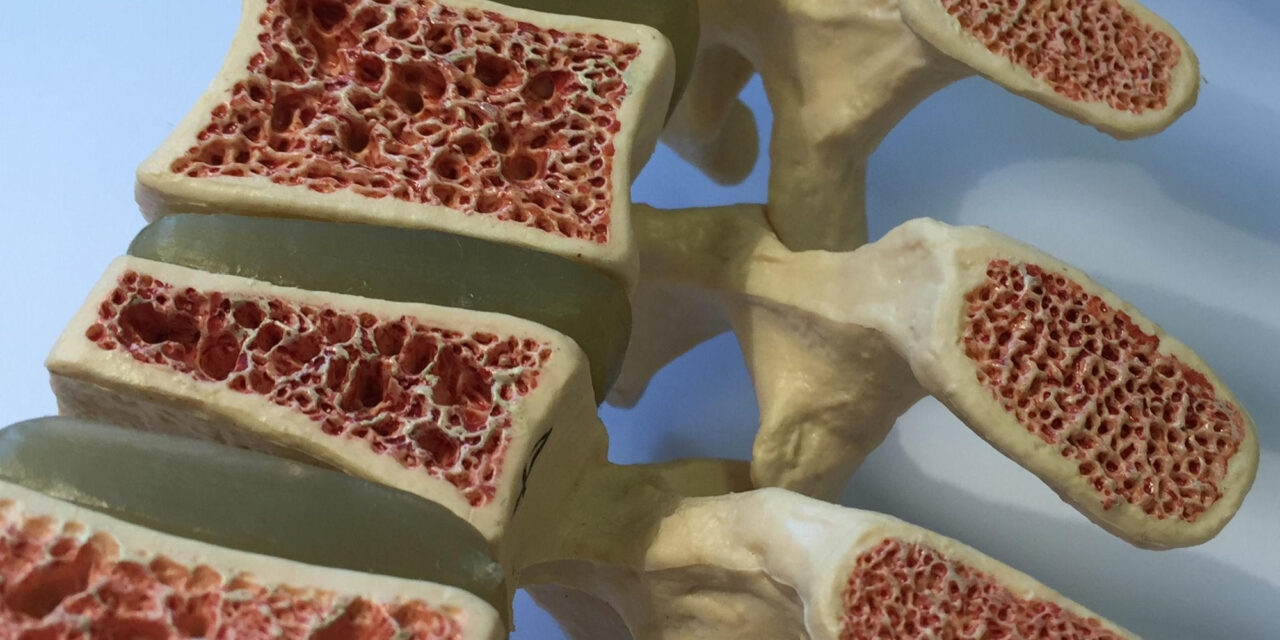
In a groundbreaking study, researchers at Harvard Medical School have dispelled a long-held belief that the gut microbiome plays a significant role in age-related bone loss, offering new insights that could revolutionize future treatments for osteoporosis. The study, published in Bone Research on November 8, 2024, has uncovered that bone deterioration in aging occurs independently of the gut microbiome, challenging current paradigms and suggesting other factors may be at play in the progression of bone loss.
Unveiling the Truth About Bone Health and the Microbiome
The research team, led by Dr. Xiaomeng You, set out to investigate whether the gut microbiome—a community of microorganisms living in the digestive tract—had an impact on bone health in aging mice. For years, studies have indicated a potential link between gut bacteria and bone metabolism, with some believing that the microbiome could influence the way bones deteriorate over time.
To explore this hypothesis, the researchers conducted a detailed study involving both germ-free mice (which had no gut microbiota) and mice colonized with a normal microbiome. Over a period of 21 months, they observed the effects of aging on bone health in both groups.
The results were surprising: both the germ-free and microbiome-colonized mice exhibited similar rates of bone loss, debunking the theory that gut microbes play a major role in age-related bone deterioration. The researchers discovered that, despite changes in microbial composition and function—such as an increase in amino acid and protein biosynthesis—these shifts had no detectable impact on the health of the bones. Even when microbiota from young or old donors was transplanted into germ-free mice, no significant changes in bone health occurred.
Implications for Osteoporosis Treatment
This discovery has profound implications for the treatment of osteoporosis, a disease characterized by weakened bones and an increased risk of fractures, especially among older adults. Osteoporosis is a global health concern, with current treatments often involving expensive drugs that carry side effects and accessibility challenges. Given the emerging belief that the microbiome might influence bone health, many had speculated that targeting gut bacteria could offer a novel treatment pathway. However, the findings from Harvard’s study suggest that microbial solutions may not be the answer.
Dr. You and her team emphasize that, while the microbiome continues to play a critical role in various aspects of human health, its influence on bone health during aging is limited. This new understanding will likely shift the focus of future osteoporosis research and treatment toward other factors, such as genetic, hormonal, and environmental contributors, which may be more significant in the regulation of bone metabolism.
“The findings challenge a long-standing theory about the role of gut bacteria in bone health,” Dr. You explained. “By redirecting our attention to other biological pathways, we open up new possibilities for more effective treatments for osteoporosis.”
A Shift in Focus for Future Research
The study’s results signal a potential turning point in the approach to osteoporosis research and treatment. As scientists begin to investigate alternative mechanisms of bone loss, new therapies may emerge that address the underlying causes of the condition, rather than relying on microbial modulation.
Despite the surprising findings, researchers stress the importance of continuing to explore the microbiome’s role in other health areas. The complexity of gut bacteria and its interactions with the body remain a rich field of study, but for now, the path forward for osteoporosis may lie in understanding the other factors that contribute to bone deterioration with age.
Conclusion
The Harvard team’s work provides critical insights into the science of aging and bone health, shedding light on the intricate factors that influence osteoporosis and challenging previous assumptions about the microbiome’s role. By shifting the focus away from microbial solutions, the scientific community can better address the pressing need for effective treatments to combat the global osteoporosis epidemic.
As researchers continue to unravel the biological mysteries behind bone health, this study lays the groundwork for more targeted therapies that could transform the care and well-being of aging populations worldwide.
Reference:
“Bone loss with aging is independent of gut microbiome in mice” by Xiaomeng You, Jing Yan, Jeremy Herzog, Sabah Nobakhti, Ross Campbell, Allison Hoke, Rasha Hammamieh, R. Balfour Sartor, Sandra Shefelbine, Melissa A. Kacena, Nabarun Chakraborty, and Julia F. Charles, Bone Research, November 8, 2024. DOI: 10.1038/s41413-024-00366-0.
This work was supported by NIH grants R01 AG046257 (JFC), the Orthopaedic Scholar Fund, the Department of Orthopaedic Surgery, Brigham and Women’s Hospital, P30 AR075042, and the Joint Biology Consortium funded by P30-AR070253. Gnotobiotic studies were performed at the National Gnotobiotic Rodent Resource Center at the University of North Carolina at Chapel Hill, funded by P40-OD010995, P30-DK034987, and the Crohn’s and Colitis Foundation.