A recent study has explored the intriguing connection between tau filaments and extracellular vesicles (EVs) in Alzheimer’s disease (AD), revealing potential new avenues for therapeutic strategies. The research, published in Nature Neuroscience, highlights how tau proteins, a hallmark of AD and other neurodegenerative diseases, are selectively packaged within EVs, potentially playing a crucial role in the progression of the disease.
Alzheimer’s disease, characterized by progressive memory loss and cognitive decline, is associated with the accumulation of tau protein in the brain. Tau is a microtubule-associated protein that stabilizes the internal structure of neurons by binding to microtubules, helping to transport essential molecules within the cell. However, in Alzheimer’s disease, tau accumulates abnormally, forming twisted filaments that disrupt normal cellular function.
Recent studies have suggested that tau may interact with extracellular vesicles—small, membrane-bound particles that carry molecules and transport them between cells. These vesicles have been implicated in various cellular processes, including communication between neurons, but their role in tau propagation has remained unclear.
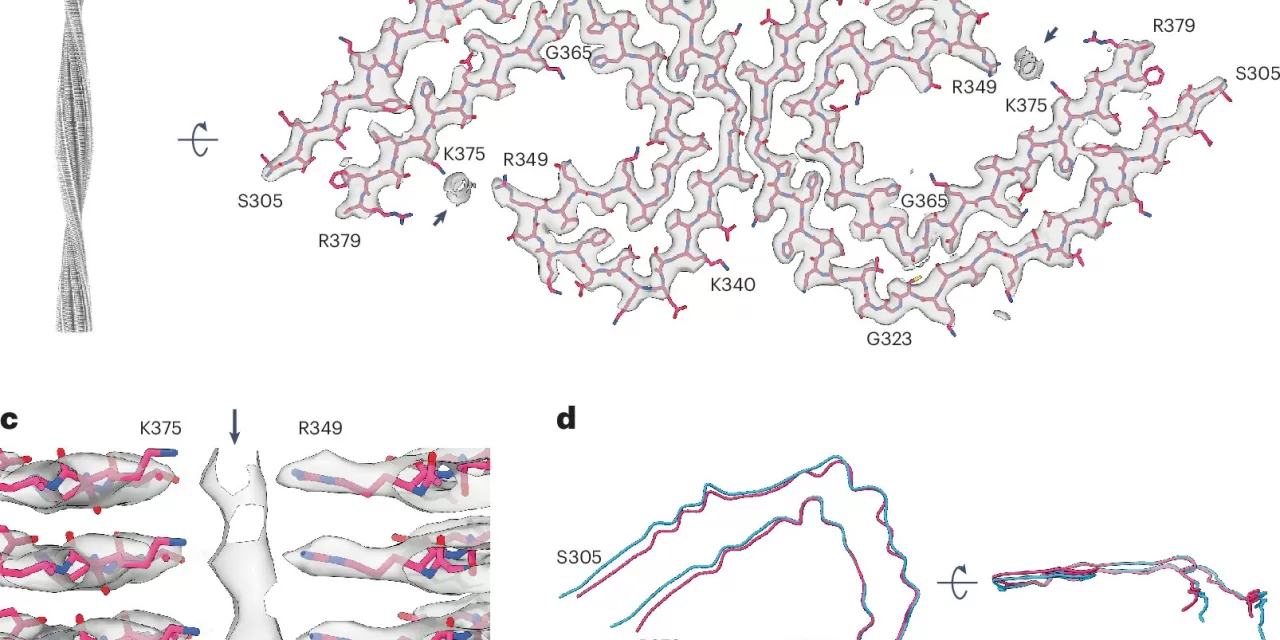
To explore this connection, a team of researchers from the UK Dementia Research Institute at University College London, the Medical Research Council Laboratory of Molecular Biology, and other institutions employed advanced techniques to study EVs derived from the brains of deceased individuals with Alzheimer’s. The team used methods such as quantitative mass spectrometry, cryo-electron tomography, and single-particle cryo-electron microscopy to examine the interactions between tau filaments and EVs in detail.
Their findings uncovered that tau filaments, primarily composed of truncated tau, were selectively packaged into EVs enriched with endo-lysosomal proteins. Moreover, they identified molecules that tethered tau filaments to the EV membranes, indicating a highly specific process of tau packaging and transport.
“Our findings suggest that tau filaments are not only packaged within EVs but are also tethered to the EV membrane, which could have significant implications for understanding how tau spreads and how it may contribute to the progression of Alzheimer’s disease,” said Stephanie L. Fowler, a lead author of the study.
This discovery provides new insights into the prion-like behavior of tau, a phenomenon where misfolded tau proteins spread from one cell to another, exacerbating disease progression. The selective packaging of tau within EVs may play a role in this process, suggesting that targeting this interaction could offer new therapeutic opportunities.
Fowler and her colleagues believe their findings will guide future research into the molecular mechanisms behind EV-mediated secretion of tau, potentially leading to novel approaches for slowing the progression of Alzheimer’s disease. The team envisions that targeting EV-associated tau could serve as both a therapeutic strategy and a biomarker for diagnosing and monitoring AD.
“We hope that our research will open up new directions for treatment strategies focused on tau and extracellular vesicles,” said Fowler. “Understanding the relationship between tau filaments and EVs could offer important clues for combating Alzheimer’s and other neurodegenerative disorders.”
As research into tau and EVs continues, this groundbreaking study marks a step forward in unraveling the complex molecular processes underlying Alzheimer’s disease and points to the potential for future therapeutic breakthroughs.
For more information, the study can be accessed in Nature Neuroscience (2024), DOI: 10.1038/s41593-024-01801-5.