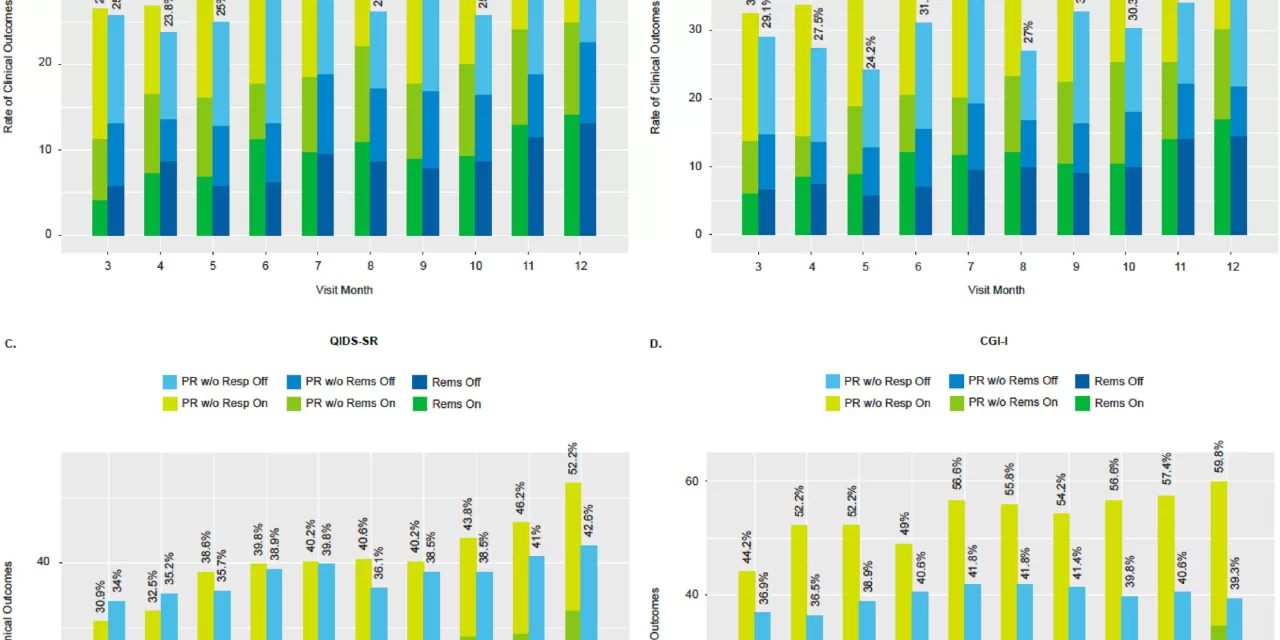
A groundbreaking clinical trial led by Washington University School of Medicine in St. Louis has found that vagus nerve stimulation (VNS) significantly improves depressive symptoms, quality of life, and daily functioning in individuals with severe, treatment-resistant depression. The study, published in Brain Stimulation, involved nearly 500 participants across 84 U.S. sites.
The trial, which is part of the larger RECOVER study, focused on individuals who had not responded to previous treatments, including medication and other therapies. Many participants had been battling depression for years, with an average of 13 unsuccessful treatments prior to the trial. The findings offer new hope for those whose conditions have remained unmanageable.
Participants in the study were implanted with a VNS device, which stimulates the left vagus nerve, a key pathway between the brain and internal organs. While half of the participants received active stimulation, the other half had their devices turned off during the control period. Over the course of 12 months, researchers tracked various measures of depression, quality of life, and daily functionality.
Results revealed that participants with activated devices experienced significant improvements in depressive symptoms, reported higher quality of life, and showed better ability to perform daily tasks. Although the primary outcome measure did not show a significant difference, secondary assessments demonstrated substantial benefits of VNS therapy.
“These patients have been extremely ill for a long time, and many have tried numerous treatments with no relief,” said Dr. Charles R. Conway, the study’s principal investigator and professor of psychiatry at WashU Medicine. “Despite their prolonged suffering, the improvements observed in both their mental health and daily functioning are truly remarkable.”
The study highlights a potential solution for patients who have been “paralyzed by life” due to their depression, according to Dr. Conway. The therapy could make a profound difference in allowing these individuals to regain the ability to engage with everyday life and loved ones.
Vagus nerve stimulation has been FDA-approved for treatment-resistant depression for nearly 20 years, but it remains inaccessible for many due to high costs and limited insurance coverage. The RECOVER study aims to provide the necessary data to persuade the Centers for Medicare and Medicaid Services (CMS) to cover the device and implantation costs, which could expand access to this potentially life-changing therapy.
The VNS Therapy device, manufactured by LivaNova U.S., Inc., involves implanting a small pacemaker-like device under the skin in the chest, with a wire connecting it to the vagus nerve in the neck. The device emits electrical pulses that stimulate brain areas associated with mood regulation. This approach has been shown to provide gradual improvements over the course of treatment, with the most significant progress occurring during the latter part of the trial.
Though the study’s primary outcome measure did not show a clear distinction between the two groups, many participants with activated devices reported feeling improvements that went beyond simple symptom relief, such as enhanced quality of life and functionality. “These patients are not just saying they feel slightly better. They are experiencing meaningful improvements in their ability to live their lives,” said Conway.
Participants in the trial will continue to be monitored for the next four years to assess the long-term effects of the therapy. Researchers are also working to identify factors that may predict the best candidates for VNS treatment.
The results of this study provide a beacon of hope for individuals suffering from severe depression, a condition that can be debilitating and resistant to traditional treatments.
For more details, refer to the papers by Charles R. Conway et al. and A. John Rush et al. published in Brain Stimulation (2024).