Malaria, a disease that claims over 600,000 lives annually—primarily African children under five—remains a persistent global health crisis. However, groundbreaking research published in Nature offers new hope in the fight against severe malaria by harnessing natural human antibodies to target its most virulent forms.
The study, conducted by an international team from EMBL Barcelona, the University of Texas, the University of Copenhagen, and The Scripps Research Institute, identifies antibodies capable of neutralizing key proteins that cause severe complications of malaria, including cerebral malaria. These findings could pave the way for novel vaccines and therapies.
Malaria’s Deadly Mechanism
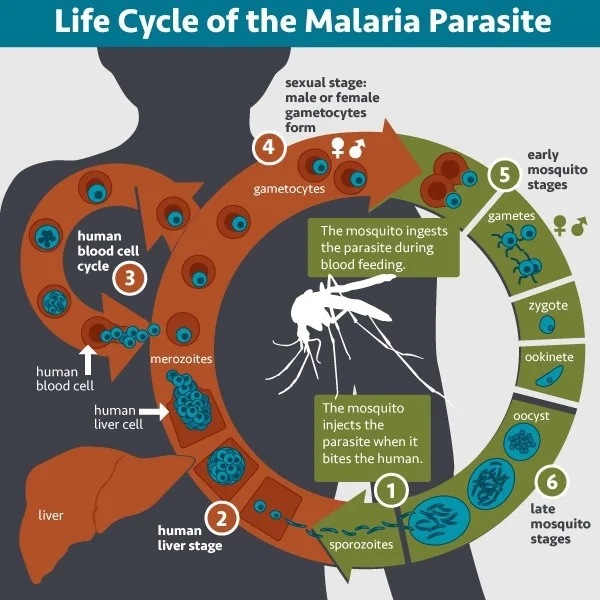
Severe malaria is caused by Plasmodium falciparum, a parasite that alters red blood cells to adhere to tiny blood vessels, especially in the brain. This blockage disrupts blood flow, leading to swelling and potentially fatal conditions such as cerebral malaria.
At the heart of this process is a family of proteins known as PfEMP1, found on the surface of infected red blood cells. Certain PfEMP1 proteins bind to a human protein called EPCR on blood vessel walls, causing vascular damage and life-threatening complications.
A Path to Immunity
Researchers have long observed that children in malaria-endemic regions gradually develop immunity as they age, with adults rarely experiencing severe symptoms. This immunity is believed to stem from antibodies targeting PfEMP1. However, PfEMP1’s variability has made it a challenging target for vaccine development.
Dr. Maria Bernabeu, co-senior author and Group Leader at EMBL Barcelona, explained:
“We were hesitant about whether we could identify a single antibody that could recognize them all.”
Using advanced immunological screening techniques developed at the University of Texas, the team identified two human antibodies that broadly neutralize diverse PfEMP1 variants by targeting a conserved region called CIDRα1, which interacts with EPCR.
Innovative Disease Modeling
Testing the efficacy of these antibodies in living systems posed a challenge, as malaria’s human-specific proteins differ from those found in other animals. To overcome this, researchers developed a laboratory model mimicking human blood vessels.
Using organ-on-a-chip technology, the team recreated 3D brain microvessels and introduced live malaria-infected blood. When the antibodies were added, they effectively blocked infected cells from adhering to vessel walls, preventing the hallmark blockages of severe malaria.
“It was striking to see the inhibition readily apparent by eye,” said Dr. Viola Introini, co-first author and postdoctoral fellow at EMBL Barcelona.
The Path Forward
Further structural and immunological analysis revealed that these antibodies recognize three conserved amino acids in CIDRα1, highlighting a potential mechanism of natural immunity to severe malaria.
Dr. Bernabeu emphasized the significance of these findings:
“This study opens the door to new ways of protecting people from severe malaria, like a vaccine or other treatments.”
The research exemplifies the power of international collaboration and interdisciplinary innovation. By combining tissue engineering with immunology, the team has provided a platform for advancing malaria research and accelerating vaccine development.
As efforts continue, these findings represent a vital step toward reducing malaria’s devastating toll.
For more information, refer to the original study: Evelien Bunnik, Broadly inhibitory antibodies to severe malaria virulence proteins, Nature (2024). DOI: 10.1038/s41586-024-08220-3.