Researchers Uncover Early “Quiet” Brain Changes in Alzheimer’s Disease
A groundbreaking study has reshaped our understanding of how Alzheimer’s disease affects the brain, suggesting that the disease unfolds in two distinct phases, or “epochs.” This finding diverges from the traditional model, which posits that Alzheimer’s progresses gradually through various stages characterized by increasing cell death, inflammation, and the accumulation of harmful proteins such as amyloid plaques and tau tangles.
The study, led by researchers Mariano I. Gabitto, Ph.D., and Kyle J. Travaglini, Ph.D., from the Allen Institute in Seattle, suggests that many of the hallmark changes associated with Alzheimer’s occur rapidly during the second phase, coinciding with the onset of memory loss and other cognitive impairments. Before this accelerated stage, the disease operates more subtly, with slow and “quiet” changes happening during the first phase.
First Phase: Gradual, Silent Changes
In the initial phase, before symptoms manifest, the brain undergoes gradual changes that include the slow buildup of amyloid plaques, the activation of the brain’s immune response, and damage to myelin—the insulating layer around neurons responsible for transmitting signals. In a surprising twist, researchers also found that Alzheimer’s significantly affects a type of neuron known as somatostatin (SST) inhibitory neurons. These neurons, which send calming signals to other brain cells, were found to be more vulnerable in the early stages of the disease than previously thought. Traditionally, scientists believed Alzheimer’s primarily targeted excitatory neurons, which transmit activating signals.
The loss of SST inhibitory neurons may play a critical role in disrupting the brain’s neural circuits, the researchers hypothesized, potentially triggering the larger-scale changes that lead to Alzheimer’s later symptoms.
Second Phase: Rapid Deterioration
The second phase of Alzheimer’s is marked by a much faster progression of brain damage, leading to noticeable cognitive symptoms like memory problems. This phase involves more widespread neuronal death, increased inflammation, and a sharp rise in the accumulation of amyloid plaques and tau tangles—the protein buildups long associated with the disease.
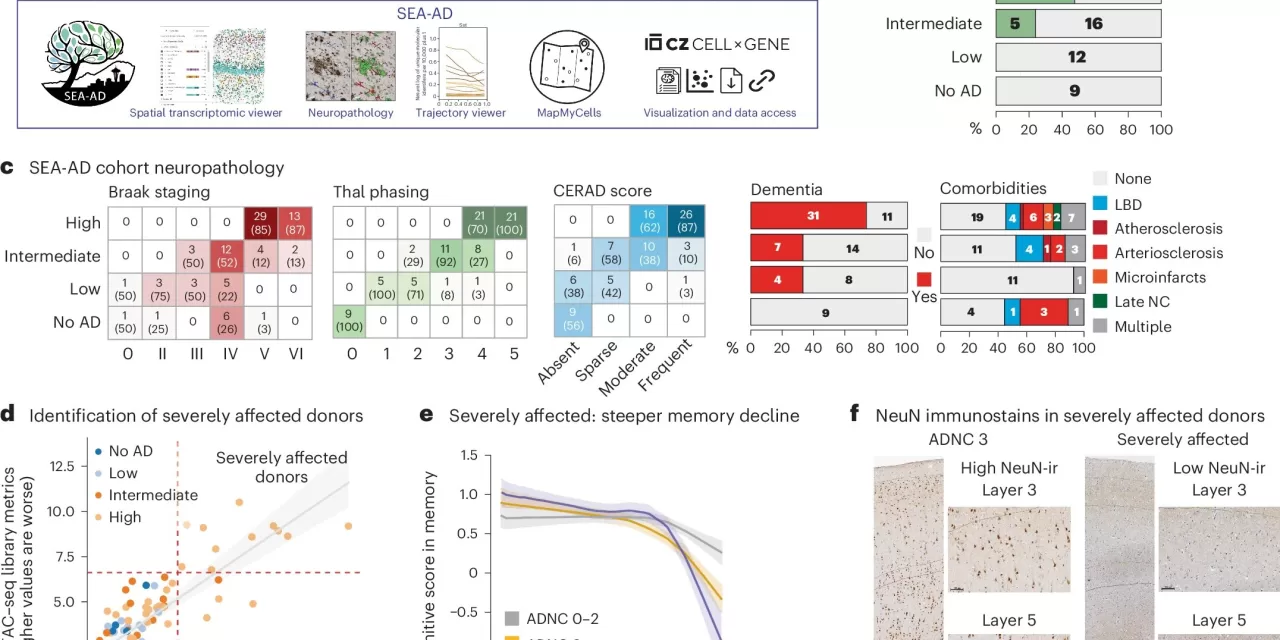
The researchers utilized advanced tools developed under the NIH’s Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative’s Cell Census Network (BICCN) to study over 3.4 million brain cells. Brain samples from individuals at different stages of Alzheimer’s were analyzed as part of the Seattle Alzheimer’s Disease Brain Cell Atlas (SEA-AD), using tissue from the University of Washington’s Alzheimer’s Disease Research Center.
Astrocytes and Resilience to Alzheimer’s
The study also aligned with recent findings from MIT researchers, which identified a gene called REELIN as a potential factor in the vulnerability of certain neurons to Alzheimer’s. The MIT study also highlighted the role of astrocytes—star-shaped cells in the brain—which may provide some resilience against the disease’s harmful effects. This raises the possibility that astrocytes could be key players in future therapies aimed at slowing the disease’s progression.
Future Impact on Alzheimer’s Treatment
“This research demonstrates how powerful new technologies provided by the NIH’s BRAIN Initiative are changing the way we understand diseases like Alzheimer’s,” said John Ngai, Ph.D., director of The BRAIN Initiative. “With these tools, scientists were able to detect the earliest cellular changes to the brain to create a more complete picture of what happens over the entire course of the disease.”
The detailed cellular map produced by this research has the potential to revolutionize the approach to Alzheimer’s diagnostics and treatment. By pinpointing the distinct phases of the disease, scientists and drug developers may now have the opportunity to create treatments that target specific stages, potentially halting or slowing progression before the more severe symptoms set in.
The findings, published in Nature Neuroscience, represent a major step forward in Alzheimer’s research, offering hope for earlier interventions and more effective treatments in the future.
Reference:
Mariano I. Gabitto et al, Integrated multimodal cell atlas of Alzheimer’s disease, Nature Neuroscience (2024). DOI: 10.1038/s41593-024-01774-5.