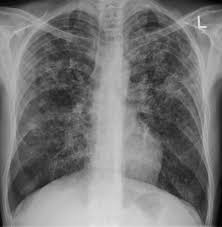
Jakarta, Indonesia – In a bid to combat the alarming rise in tuberculosis (TB) cases, Indonesia is fast-tracking the development of a new, domestically produced TB vaccine. This initiative is part of a broader strategy to reduce the national TB burden, which currently claims an average of 150,000 lives annually.
Minister of Health Budi Gunadi Sadikin announced during a virtual press conference on Friday that the new vaccine has entered the clinical trial stage. “The renewal of the TB vaccine was due to the fact that the existing vaccine had been outdated,” Sadikin stated. He emphasized the need to expedite the vaccine’s launch, a priority set by President-elect Prabowo Subianto, who is scheduled to take office in October.
Tuberculosis remains a significant public health challenge worldwide, and Indonesia is no exception. According to the 2023 Global Tuberculosis Report by the World Health Organization (WHO), Indonesia recorded 1,060,000 TB cases in the past year, translating to a rate of 385 cases per 100,000 people. The Indonesian government is committed to reducing this rate to 297 cases per 100,000 people by 2024, with a long-term goal of reaching just 65 cases by 2030.
“We want to focus on prevention, not just on treating those who are already sick,” Sadikin explained. “This health strategy is more cost-effective and will significantly improve the quality of life for our people. Our goal is to keep our society healthy.”
In addition to the vaccine development, Indonesia recently launched a new TB screening tool, the Portable X-Ray, designed to accelerate the detection of TB patients and symptoms. This tool is expected to play a crucial role in the country’s efforts to control the spread of the disease.
As Indonesia pushes forward with these initiatives, the hope is that the combination of a new vaccine and enhanced screening will help curb the TB epidemic and improve public health outcomes across the nation.