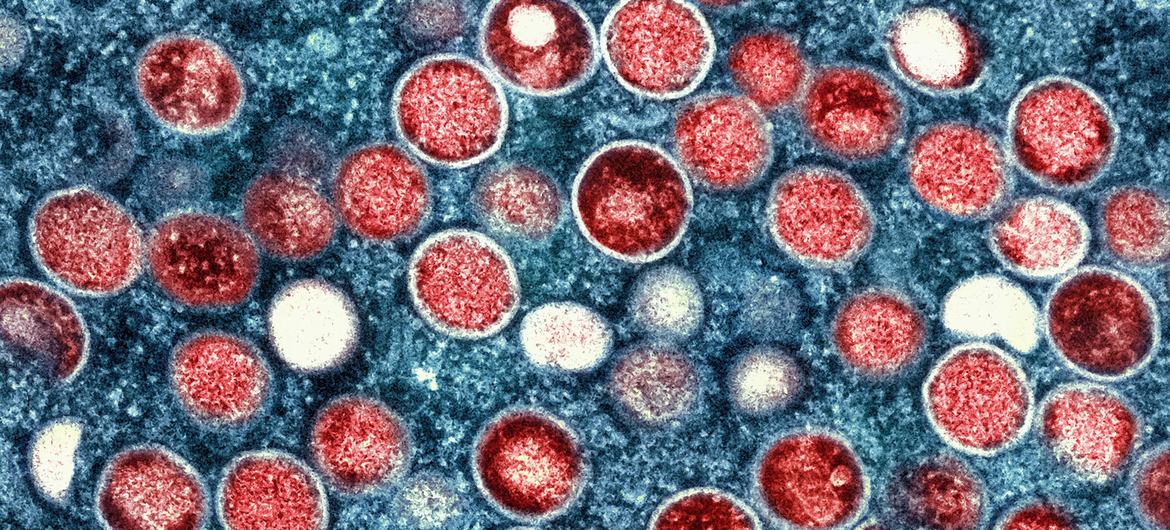
Geneva, August 14, 2024 — The World Health Organization (WHO) is set to meet this Wednesday to determine whether the escalating mpox outbreak in central Africa warrants a declaration of Public Health Emergency of International Concern (PHEIC). This move could mark only the eighth PHEIC declaration by the WHO, reflecting the severity of the current situation.
The focus of concern has shifted from the previously less lethal Clade 2 variant of mpox, which prompted the last PHEIC declaration in May 2022, to the more dangerous Clade 1b. Unlike its predecessor, Clade 1b is transmitted directly between humans through sexual contact, heightening the risk of rapid spread.
In a concurrent development, the Africa Centres for Disease Control and Prevention (Africa CDC) plans to declare a Public Health Emergency of Continental Security. This unprecedented measure will enable African Union countries to coordinate their response more effectively, aiming to curb the epidemic’s spread.
Both emergency declarations are crucial for controlling a virus that poses a significant risk of evolving into a global pandemic. A PHEIC could expedite the procurement of mpox vaccines for African countries, bypassing the often lengthy national licensing processes and increasing access to vaccine supplies beyond what has been donated.
However, logistical challenges persist. For example, the U.S. has donated 50,000 vaccine doses to the Democratic Republic of Congo (DRC), but these are not expected to arrive for at least two more months. Ensuring effective distribution will require addressing issues related to transportation, refrigeration, trained health workers, and other essential resources.
Jean Kaseya, head of Africa CDC, highlighted that the continental emergency declaration would facilitate joint negotiations for existing and new mpox vaccines, including an mRNA vaccine being developed by BioNTech in Rwanda.
WHO Director-General Tedros Adhanom Ghebreyesus explained that the decision to convene the expert committee was driven by the virus’s spread beyond the DRC and its potential for further international transmission.
Rosamund Lewis, WHO’s emergency manager for mpox, noted that Clade 1b is showing signs of a significant epidemic. “It’s climbing disproportionately faster than cases of Clade 1a elsewhere in the DRC,” she said. Although Clade 1a is also increasing, it involves animal transmission, which limits its pandemic potential compared to the human-to-human spread of Clade 1b.
Vaccines remain a key tool in controlling the outbreak. Two vaccines—one from Bavarian Nordic in Denmark and another from KM Biologics in Japan—are commercially available and could help contain the virus. Recent trials initiated by the Coalition for Epidemic Preparedness Innovations (CEPI) in the DRC aim to assess if vaccination post-exposure can prevent illness and further spread.
Despite these efforts, the lack of a WHO emergency designation complicates the vaccine procurement process. The WHO has initiated the Emergency Use Listing process for both vaccines, but this will take additional time as it involves approval, negotiation, and importation stages.
The DRC alone estimates a need for 10 million doses to manage both Clade 1b and protect against Clade 1a. The production speed and capacity of vaccines, such as those grown in hen’s eggs by Bavarian Nordic, remain unclear, adding to the urgency of the situation.
As the world awaits the WHO’s decision, the coordination between global and continental health authorities will be pivotal in combating this pressing public health challenge.