July 6, 2024 – A new, deadly strain of mpox has emerged in the Democratic Republic of Congo (DRC), raising fears of a potential global outbreak. This novel strain, which is sexually transmitted, first triggered an outbreak in September in the southeast of the country and has since spread to another province.
Health officials are deeply concerned that the virus may have already crossed borders and could be spreading undetected, similar to the strain responsible for the global epidemic in 2022. However, the new strain appears to be significantly more lethal.
The Urgent Need for Vaccines
To combat this threat, the DRC and neighboring countries urgently need to access smallpox vaccines, which are effective against mpox. The DRC recently secured regulatory approval to use donated vaccines, a crucial step in containment efforts. In April, USAID offered 50,000 doses of a vaccine from the U.S. stockpile, but this is insufficient for the population at risk.
While the DRC can now accept and use such donations, acquiring the necessary number of doses through agencies like GAVI remains a challenge. The vaccines, costing $200 for a two-dose course, require prequalification from the World Health Organization (WHO) for use against mpox, a lengthy process that has not yet begun.
Emergency Measures and Global Preparedness
A WHO Emergency Use Listing would facilitate vaccine purchases, but this is typically issued only when a Public Health Emergency of International Concern is declared. The previous declaration for mpox was lifted in May 2023 after vaccination and behavioral changes reduced case numbers. However, as the virus spreads into crowded refugee camps, an emergency vaccine authorization might soon be necessary.
GAVI plans to establish a global stockpile of mpox vaccines, and the WHO’s Strategic Framework for mpox aims for a rapid global vaccine deployment mechanism by 2026. These measures depend on either the WHO’s slow approval process or emergency use policies to provide lower-income countries with access to vaccines.
The Threat of the New Strain
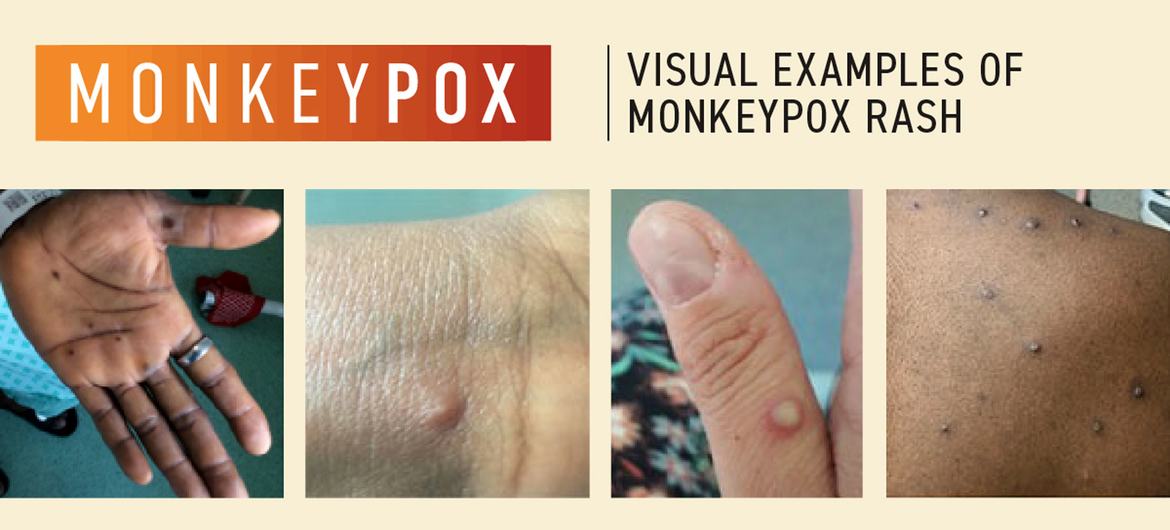
The new sexually transmitted strain, circulating exclusively in humans, poses a significant threat of exponential spread, reminiscent of the 2022 epidemic that affected over 96,000 people in 116 countries. This strain’s symptoms include a rash resembling blisters, fever, aches, and swollen glands.
Historically, mpox cases in Africa were contracted from small mammals, but the new DRC strain and the West African strain from 2022 have mutated to sustain sexual transmission exclusively among humans, posing a global threat.
Recent Developments and Ongoing Challenges
On June 24, the DRC approved two mpox vaccines after extensive regulatory efforts, making it the second African country to do so. In theory, this approval means the new strain can be contained if sufficient vaccinations and other health measures are implemented.
However, achieving this in the DRC is challenging due to ongoing armed violence and frequent cross-border movements. Cases have already been reported in a refugee camp in Goma, North Kivu, raising concerns about spread to neighboring Uganda, Rwanda, and Burundi.
The success of containment efforts hinges on the rapid acquisition and distribution of vaccines across the country. The DRC’s healthcare system is already strained, with rising cases and high mortality rates from the normal type of mpox caught from animals.
A Global Health Priority
Mpox, closely related to smallpox, has seen a resurgence due to the cessation of widespread smallpox vaccination in 1980. The animal-borne Clade II virus mutated to Clade IIb, which spread human-to-human and led to the 2022 epidemic. Now, the Clade I virus in the DRC has given rise to Clade Ib, which also spreads sexually and has a potentially higher death rate.
Jean Kaseya, head of the African Centres for Disease Control and Prevention, emphasized on June 13 that an mpox outbreak anywhere is a threat everywhere, calling for swift action to improve Africa’s access to vaccines, diagnostics, and antiviral drugs.
To effectively respond to this urgent situation, enabling WHO emergency use listing of the vaccine is a critical step for a rapid and coordinated global response.