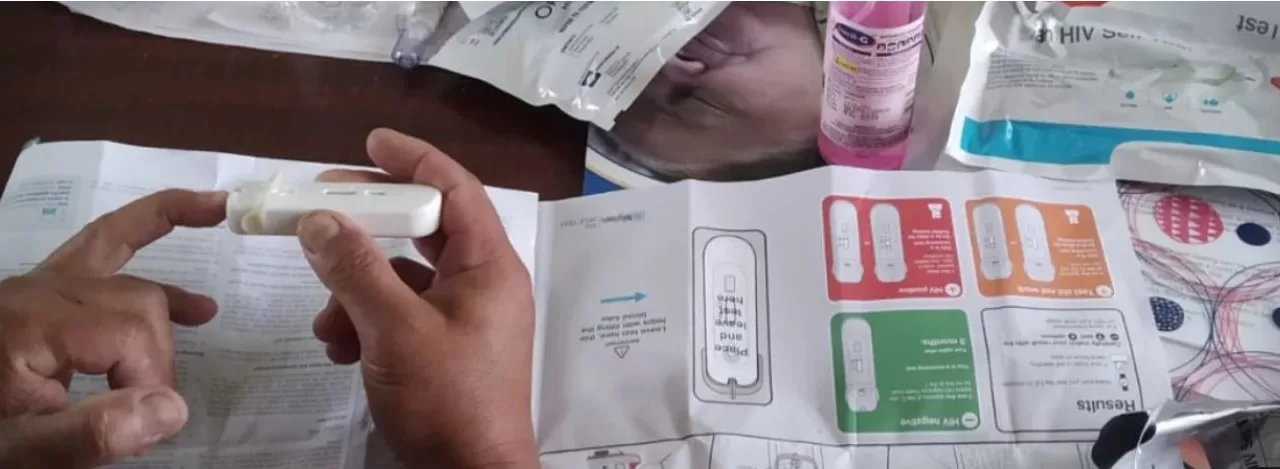
A pioneering HIV vaccine candidate developed at the Duke Human Vaccine Institute (DHVI) has demonstrated the ability to trigger low levels of elusive broadly neutralizing HIV antibodies in a small cohort of participants from a 2019 clinical trial. The significant findings, reported in the journal Cell on May 17, validate that a vaccine can initiate these antibodies, which are essential for combating various HIV strains, within a matter of weeks.
The vaccine targets a stable area on the HIV-1 outer envelope known as the membrane proximal external region (MPER). This region remains consistent even as the virus mutates, making it an ideal target for antibodies that can block infection by multiple circulating HIV strains.
“This work is a major step forward as it shows the feasibility of inducing antibodies with immunizations that neutralize the most difficult strains of HIV,” said Dr. Barton F. Haynes, senior author and director of DHVI. “Our next steps are to induce more potent neutralizing antibodies against other sites on HIV to prevent virus escape. We are not there yet, but the way forward is now much clearer.”
The study analyzed data from a phase 1 clinical trial involving twenty healthy, HIV-negative participants. Of these, fifteen received two of four planned doses of the investigational vaccine, while five received three doses. The vaccine demonstrated a 95% serum response rate and a 100% blood CD4+ T-cell response rate after just two immunizations, indicating strong immune activation. Importantly, broadly neutralizing antibodies were induced after only two doses.
The trial was halted when one participant experienced a non-life-threatening allergic reaction, similar to rare incidents seen with some COVID-19 vaccinations. The reaction was likely due to an additive in the vaccine. Despite this, the results were encouraging, showcasing the vaccine’s ability to prompt a critical immune response quickly.
“To get a broadly neutralizing antibody, a series of events needs to happen, and it typically takes several years post-infection,” explained lead author Dr. Wilton Williams. “The challenge has always been to recreate the necessary events in a shorter space of time using a vaccine. It was very exciting to see that, with this vaccine molecule, we could actually get neutralizing antibodies to emerge within weeks.”
The researchers noted that further development is required to enhance the response and target more regions of the HIV envelope. A successful HIV vaccine will likely need to address at least three distinct regions of the virus.
“Ultimately, we will need to hit all the sites on the envelope that are vulnerable so that the virus cannot escape,” said Haynes. “But this study demonstrates that broadly neutralizing antibodies can indeed be induced in humans by vaccination. Now that we know that induction is possible, we can replicate what we have done here with immunogens that target the other vulnerable sites on the virus envelope.”
In addition to Haynes and Williams, the study’s authors include S. Munir Alam, Gilad Ofek, Nathaniel Erdmann, David Montefiori, Michael S. Seaman, Kshitij Wagh, Bette Korber, Robert J. Edwards, Katayoun Mansouri, Amanda Eaton, Derek W. Cain, Mitchell Martin, Robert Parks, Maggie Barr, Andrew Foulger, Kara Anasti, Parth Patel, Salam Sammour, Ruth J. Parsons, Xiao Huang, Jared Lindenberger, Susan Fetics, Katarzyna Janowska, Aurelie Niyongabo, Benjamin M. Janus, Anagh Astavans, Christopher B. Fox, Ipsita Mohanty, Tyler Evangelous, Yue Chen, Madison Berry, Helene Kirshner, Elizabeth Van Itallie, Kevin Saunders, Kevin Wiehe, Kristen W. Cohen, M. Juliana McElrath, Lawrence Corey, Priyamvada Acharya, Stephen R. Walsh, and Lindsey R. Baden.
This groundbreaking study opens new avenues for the development of a comprehensive HIV vaccine, bringing renewed hope to the fight against the virus.