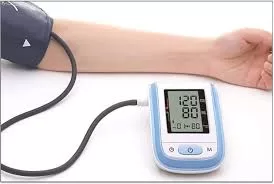
A new study published in JAMA Network Open suggests that self-monitoring of blood pressure (BP) combined with medication management may offer superior outcomes for individuals battling hypertension compared to standard care.
Conducted as a secondary analysis of a randomized clinical trial in Valencia, Spain, the study focused on patients aged 40 and above with uncontrolled hypertension between 2017 and 2020. Led by Gabriel Sanfélix-Gimeno, PhD, Pharm D, from the Health Services Research & Pharmacoepidemiology Unit at Fisabio Research Institute, the trial enlisted 111 patients in the intervention group and 108 in the control group.
Patients in the intervention group were provided with educational materials and equipped with home BP monitors, along with instructions for self-monitoring and medication adjustments as necessary, without direct consultation with healthcare professionals. Conversely, the control group received standard care, including education on BP control.
After 24 months, researchers assessed BP levels, the proportion of patients achieving target BP levels, adverse events, quality of life, behavioral changes, and healthcare utilization.
Results indicated that individuals in the intervention group exhibited a lower average systolic BP reading at the end of the study period compared to those receiving standard care, with an adjusted mean difference of -3.4 mm Hg. Additionally, the intervention group showed a lower average diastolic BP reading, with an adjusted mean difference of -2.5 mm Hg. While the percentage of individuals achieving target BP levels was comparable between the two groups, researchers observed no significant differences in adverse events, healthcare utilization, behavioral changes, or quality of life.
Sanfélix-Gimeno emphasized, “These results suggest that simple, inexpensive, and easy-to-implement self-management interventions have the potential to improve the long-term control of hypertension in routine clinical practice.”
However, the study acknowledged limitations, including loss to follow-up due to COVID-19 restrictions, potential biases stemming from the unblinded nature of the trial, and the fact that clinicians treated both control and intervention groups. Moreover, the results may not be directly applicable to individuals with controlled hypertension, severe hypertension, or pregnant individuals, as they were not represented in the study.
Disclosure statements revealed that several authors received grants from RTI Health Solutions or personal fees from GSK and MSD outside the scope of the study. Funding for the research was provided by the Instituto de Salud Carlos III at the Spanish Ministry of Research, Innovation and Universities, the European Regional Development Fund, and the Spanish Clinical Research Network.