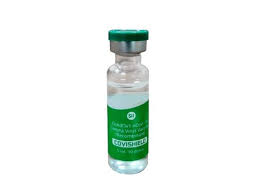
In a recent development, a plea has been filed in the Supreme Court urging the formation of a medical panel of experts supervised by a retired judge of the apex court. The purpose of this panel would be to examine the side effects and risks associated with the Covishield vaccine. This move comes in light of reports where the developer and pharmaceutical giant AstraZeneca acknowledged that its AZD1222 vaccine, widely known as Covishield, could potentially lead to low platelet counts and the formation of blood clots in “very rare” instances.
Advocate Vishal Tiwari, who lodged the application, emphasized the escalating cases of fatalities due to heart attacks, including among young individuals, and sudden collapses following Covid-19. The plea seeks a directive to the Union government to establish a Vaccine Damage Payment System, aiming to provide support to citizens who suffer severe disabilities or fatalities as a consequence of vaccination.
Citing a document presented in a UK court by the developer of the Oxford-AstraZeneca Covid vaccine, sold under the brand Covishield in India, the plea underscores the immediate need for the government’s intervention to ensure the safety and well-being of Indian citizens. It asserts that prioritized action is imperative to mitigate potential risks to the health and lives of the populace.
AstraZeneca’s recent acknowledgment during court proceedings in the UK High Court is pivotal to the plea. While the pharmaceutical giant conceded the possibility of Thrombosis with Thrombocytopenia Syndrome (TTS) occurring as a rare side effect of its vaccination, it denied a direct causal link between TTS and the vaccine at a generic level.
This plea in the Supreme Court underscores the growing concerns surrounding vaccine safety and calls for a comprehensive examination of the risks associated with Covishield. It highlights the need for proactive measures by the authorities to safeguard public health and instill confidence in vaccination efforts amidst ongoing global health challenges.