Genetic studies of diseases have long been instrumental in mapping segments of the genome responsible for driving various conditions. However, understanding how these genetic changes contribute to disease progression requires unraveling their impact on gene regulation within cell populations thought to be pivotal in driving the disease.
Enter “enhancer-gene maps” — crucial tools that link genomic regulatory regions to genes and are fundamental for comprehending disease mechanisms. Yet, constructing these maps has posed challenges due to limitations in existing experimental methods, particularly in applying the technique to rare cell populations and genes regulating specific cell types.
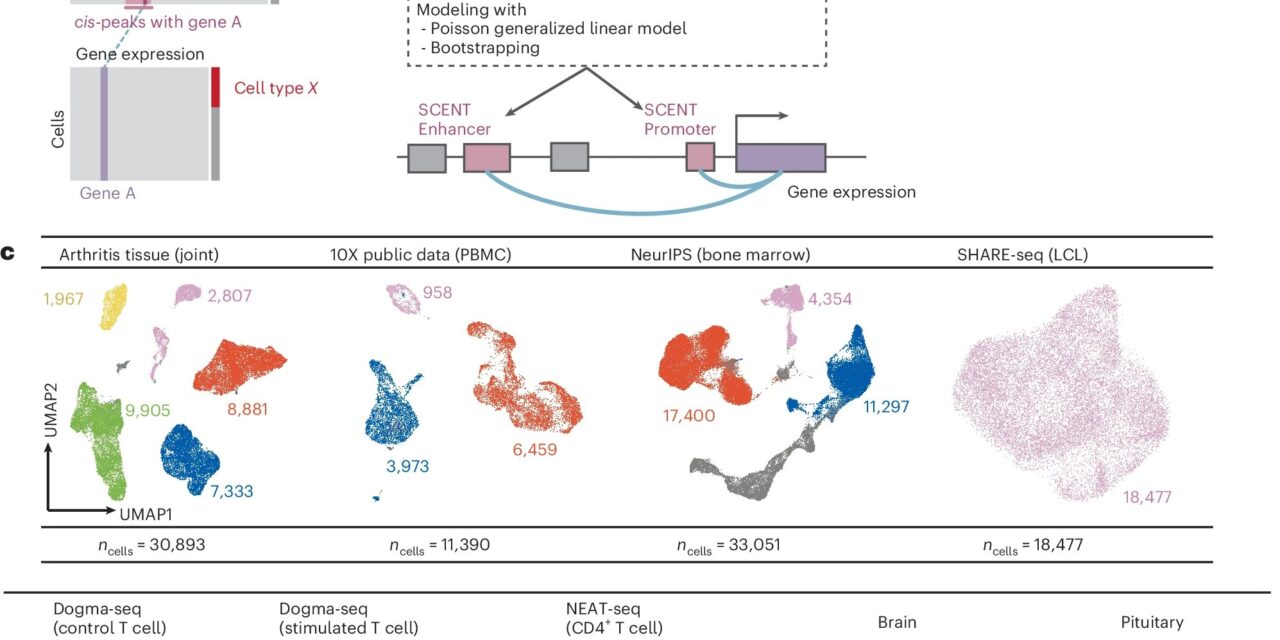
Researchers from Brigham and Women’s Hospital, a flagship institution within the Mass General Brigham health care system, have devised a groundbreaking statistical method named SCENT (single-cell enhancer target gene mapping). SCENT utilizes multimodal single-cell data to establish connections between regulatory elements and genes, thereby pinpointing likely causal gene loci for both common and rare diseases. These insights hold promise for the development of treatments across a spectrum of medical conditions.
The research team deployed SCENT across nine multimodal single-cell datasets representing diverse human tissues, encompassing immune, neuronal, and pituitary cells. Their objective was to delve into the intricacies of DNA regulation within each specific cell type.
Through meticulous analysis, they crafted 23 distinct gene-enhancer maps to explore genetic variants and expression patterns associated with 1,143 diseases and traits. Of particular note was the revelation that, for immune diseases, invaluable insights emerged not only from immune cells but also from cells residing within the affected tissues themselves.
Dr. Soumya Raychaudhuri, MD, Ph.D., of Brigham’s Division of Rheumatology, Immunology, and Inflammation, underscored the significance of this finding, stating, “For most autoimmune diseases, people assume that we need a general map of immune cells. But we find that the enhancer-gene maps of immune cells are different in affected disease tissues. We demonstrate how such a map can be used to interpret genetic data from rheumatoid arthritis and other autoimmune diseases.”
Published in the prestigious journal Nature Genetics, the study marks a significant stride toward unraveling the intricate genetic underpinnings of disease progression. By shedding light on the regulatory mechanisms governing gene expression, SCENT holds promise for advancing our understanding of various diseases and paving the way for more targeted therapeutic interventions.