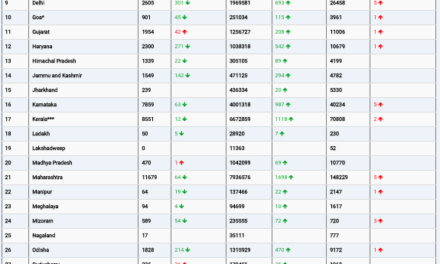
In a significant stride toward combating sepsis, the FDA has granted authorization for Prenosis, Inc.’s Sepsis ImmunoScore, an Artificial Intelligence/Machine Learning-Based Software designed to identify patients at risk for developing or already having sepsis. Sepsis, a potentially life-threatening condition triggered by the body’s extreme response to an infection, claims the lives of nearly 270,000 Americans annually, according to the Centers for Disease Control and Prevention.
The authorization marks a pivotal moment in the fight against sepsis, offering healthcare professionals a powerful tool to assess and manage patients at risk. Dr. Jeff Shuren, Director of the FDA’s Center for Devices and Radiological Health, emphasized the importance of technologies like Prenosis’ Sepsis ImmunoScore in preventing the deadly complications associated with sepsis.
“Sepsis is a serious and sometimes deadly complication. Technologies developed to help prevent this condition have the potential to provide a significant benefit to patients,” stated Dr. Shuren. “To help ensure the safety and effectiveness of software as a medical device, the FDA’s authorization of the Prenosis Sepsis ImmunoScore software establishes specific premarket and postmarket requirements for this device type, including software validation and clinical performance testing for the intended use before they are authorized for marketing.”
The Sepsis ImmunoScore software leverages patient data from electronic health records, combined with laboratory findings and clinical assessments, to facilitate risk assessment for the presence of sepsis or its progression within 24 hours of assessing a patient admitted to the emergency department or hospital, meeting certain criteria. However, it is essential to note that the software should not be the sole basis for determining the presence of sepsis.
In a related development, the FDA published a CDER Conversation highlighting the importance of postmarket surveillance and risk assessment programs to identify and evaluate adverse drug reactions and medication errors that may not have been apparent during the drug development process. Gerald J. Dal Pan, Director of CDER’s Office of Surveillance and Epidemiology (OSE), emphasized the agency’s commitment to developing and implementing processes to ensure efficient and effective postmarket safety measures.
The approval of Prenosis’ Sepsis ImmunoScore software represents a significant advancement in sepsis management, offering healthcare providers a powerful tool to identify and intervene in cases of sepsis promptly. With the FDA’s stringent premarket and postmarket requirements in place, the software is poised to make a meaningful impact in reducing the burden of sepsis and saving lives across the United States.