An Innovative Treatment for Locally Advanced or Metastatic Urothelial Carcinoma
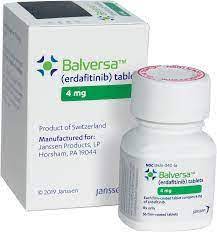
In a significant stride forward for cancer treatment, the U.S. Food and Drug Administration (FDA) has granted approval to erdafitinib (Balversa, Janssen Biotech) for adult patients battling locally advanced or metastatic urothelial carcinoma (mUC). The approval is specifically intended for individuals exhibiting susceptible FGFR3 genetic alterations, as determined by an FDA-approved companion diagnostic test.
This groundbreaking decision, announced on Friday, extends the scope of erdafitinib’s application to patients whose urothelial carcinoma has progressed after at least one line of prior systemic therapy. Notably, the FDA emphasizes that erdafitinib is not recommended as a primary treatment for patients eligible for, yet unreceived, prior PD-1 or PD-L1 inhibitor therapy.
The latest approval marks an amendment to the previous indication granted under accelerated approval. Initially, erdafitinib was greenlit for use in patients with mUC featuring susceptible FGFR3 or FGFR2 alterations after undergoing prior platinum-containing chemotherapy.
Precision Medicine at the Forefront
Erdafitinib’s approval underscores the growing significance of precision medicine in the field of oncology. Tailored to target urothelial carcinoma patients with specific FGFR3 genetic alterations, erdafitinib represents a paradigm shift in the approach to cancer treatment. The FDA emphasizes the importance of using an FDA-approved companion diagnostic test to identify patients eligible for erdafitinib therapy.
Strategic Treatment Considerations
Importantly, the FDA recommendation specifies that erdafitinib should be reserved for patients who have experienced disease progression following prior systemic therapy. This strategic use of erdafitinib optimizes its impact, offering a therapeutic avenue for those whose medical journey has encountered challenges with existing treatments.
The approval aligns with the FDA’s commitment to advancing effective and targeted therapies, acknowledging the evolving landscape of cancer research and treatment. Erdafitinib stands as a beacon of hope for patients with advanced urothelial carcinoma, providing a nuanced and targeted approach to confront the complexities of the disease.
View the full prescribing information for Balversa for comprehensive details on erdafitinib’s indications, usage, and safety information.