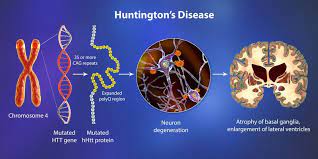
Huntington’s disease, caused by a single gene mutation, is the most prevalent neurodegenerative ailment. It manifests with motor, cognitive, and behavioral symptoms. Currently, there are no medications capable of halting or reversing the condition. However, recent research from Boston Children’s Hospital suggests a potential avenue to safeguard the brain and potentially prevent or mitigate cognitive decline.
Studies led by Beth Stevens’ team reveal that elements of the immune system, specifically complement proteins and microglia, play a role in the loss of specific synapses linking the cortex and striatum in the brain. These findings, recently published in Nature Medicine, may offer insights into other neurodegenerative conditions, such as Alzheimer’s. In 2012, Stevens’ lab was among the first to demonstrate that microglia engage in synaptic pruning during normal brain development, refining the brain’s connections. The lab also revealed that complement proteins label synapses earmarked for removal. Stevens and her team hypothesized that in diseases characterized by synaptic loss, like Alzheimer’s, schizophrenia, and Huntington’s Disease, this pruning process is abnormally reactivated.
Huntington’s Disease presented an ideal opportunity to test their theory, both due to its association with a single gene and its well-defined pathology, which affects specific brain regions and even particular synaptic connections between neurons at early disease stages. Utilizing an animal model and postmortem brain samples from Huntington’s patients, Stevens and colleagues demonstrated that complement proteins and microglia become activated very early in the illness—before cognitive and motor symptoms manifest. They target a specific vulnerable brain circuit in the pathway connecting the cortex and striatum.
The corticostriatal circuits are known to play a role in movement and learning which actions lead to positive outcomes or “reward.” The researchers observed heightened levels of complement proteins, particularly around these synapses in the striatum. Meanwhile, inputs from neurons in other brain regions that connect to the same cells were relatively unaffected. When the team blocked the complement protein C1q in their animal model—either with an antibody or by genetically removing the complement receptor CR3 on microglia—they prevented synapse loss. This intervention also forestalled cognitive deficits related to visual discrimination learning and cognitive flexibility.
Dan Wilton, PhD, the first author of the study, notes, “Some cognitive deficits tend to develop much earlier than motor defects in Huntington’s disease, and this occurs in humans, too.” The Huntington’s Disease model they studied did display some minor motor defects, which were also resolved with complement blocking strategies. Wilton and colleagues elucidated a mechanism of specificity, selective vulnerability, and what transpires in the earliest stages of Huntington’s Disease. These findings also suggest a potential biomarker: levels of innate immune molecules were elevated in the cerebrospinal fluid (CSF) of Huntington’s patients, even before the appearance of motor symptoms, correlating with a known predictor of pathological severity and disease onset.
“We’re excited by the idea that we could identify neuroimmune biomarkers to stratify people at the earliest stage and prioritize some for treatment,” says Stevens, a member of the FM Kirby Neurobiology Center at Boston Children’s and affiliate with the Broad Institute and Howard Hughes Medical Institute. “If you had clinical samples such as CSF, measuring these biomarkers could bring insight into what is happening in the brain.” Stevens believes similar mechanisms and biomarkers may apply to other neurodegenerative disorders, such as Alzheimer’s and frontotemporal dementia, which her lab is also investigating. However, most immediately, Stevens’ team aims to unravel how the huntingtin mutation leads to complement activation. While they know that specific expression of mutant huntingtin in both cortical and striatal neurons is necessary to initiate the synaptic elimination mechanism, the factors leading to the selective targeting of corticostriatal inputs remain to be determined. “Huntington’s is a nice model to tease this out,” said Stevens. “That’s a major future direction for our group.”