When combatting diseases, our immune cells must swiftly reach their intended targets. Fascinatingly, recent research conducted at the Institute of Science and Technology Austria (ISTA) reveals that immune cells actively construct their own navigation system, challenging prior assumptions about their movements. These groundbreaking findings, featured in the journal Science Immunology, expand our comprehension of the immune system and hold promise for innovative approaches to boost human immune responses.
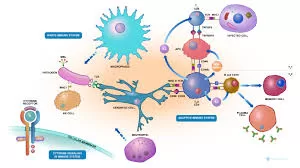
In the human body, immunological threats like pathogens and toxins can emerge anywhere. Fortunately, our immune system acts as our protective shield, employing sophisticated methods to counter these threats. Notably, during infections and inflammation, immune cells engage in coordinated collective movements. But the question remains: how do these immune cells determine their direction?
Addressing this question, a team of scientists from ISTA delved into the immune cells’ ability to collectively navigate complex environments. Dendritic cells, pivotal to our immune response, act as messengers bridging the innate and adaptive immune responses. These cells scan tissues for intruders, and upon detecting an infection, they activate and migrate to the lymph nodes, where they initiate the next steps in the immune response.
Traditionally, the migration of dendritic cells towards lymph nodes was believed to be guided by chemokines—small signaling proteins released from the lymph nodes—that establish a concentration gradient. However, novel research at ISTA challenges this conventional wisdom.
Researchers closely examined a receptor on activated dendritic cells called “CCR7.” CCR7’s primary role is to bind to a lymph node-specific molecule (CCL19), triggering subsequent immune responses. Astonishingly, the researchers discovered that CCR7 not only senses CCL19 but actively contributes to shaping the distribution of chemokine concentrations. This dual function allows immune cells to create their own guidance cues, enhancing the coordination of their collective migration.
To quantitatively understand this mechanism on a multicellular scale, the researchers collaborated with theoretical physicists who used computer simulations to replicate the experiments. The simulations revealed that dendritic cell movement not only depends on their individual responses to chemokines but also on the density of the cell population, underscoring the collective nature of this phenomenon.

Moreover, the study found that T-cells, specific immune cells responsible for destroying harmful pathogens, also benefit from this dynamic interplay to enhance their directional movement. This novel interaction principle between cell populations opens new avenues for exploration.
These discoveries represent a paradigm shift in our understanding of cell movement within the human body. Immune cells, contrary to prior beliefs, not only react to chemokines but actively shape their environment by consuming these chemical signals. This dynamic regulation of signaling cues provides an elegant strategy for guiding their movement and that of other immune cells.
The implications of this research are significant for our understanding of immune responses within the body. By unraveling these mechanisms, scientists may have the potential to design new strategies to enhance the recruitment of immune cells to specific locations, such as tumor cells or infection sites.