Formulations of bedaquiline and delamanid are now listed for use in children with TB of all ages, in line with the latest WHO recommendations.
Ethionamide has now been added to the medicines that can be used to treat people with drug-susceptible TB, given its role in the 6-month regimen for drug-susceptible TB meningitis up to 19 years of age. Additional changes include the removal of oral liquid formulations of ethambutol, isoniazid and pyrazinamide, given that dispersible tablet formulations of these medicines are now widely available; the removal of a powder preparation of linezolid that could be combined with a liquid for oral use, given that dispersible tablets are preferred and available at a lower price, with more convenient packaging.; and the inclusion of a new formulation of p-aminosalicylic acid (as p-aminosalicylate sodium), replacing the previously listed one which has been discontinued.
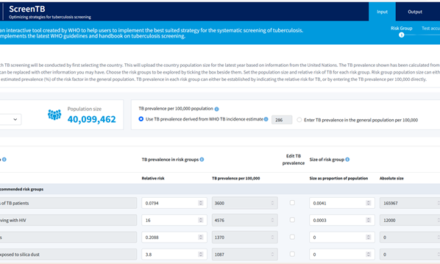
The revised list provides a comprehensive set of quality-assured TB medicines for TB preventive treatment as well as for the treatment of drug susceptible and drug resistant TB.
Essential medicines are defined as medicines that meet the priority health care needs of a population, including the treatment and prevention of TB. WHO recommends that essential medicines are available in health systems at all times, in appropriate dosage forms, of assured quality and at prices that individuals and health systems can afford.
WHO publishes a Model List of Essential Medicines (EML) and a Model List of Essential Medicines for Children (EMLc), and updates them every two years. The medicines on the lists are selected based on disease prevalence, public health relevance, evidence of efficacy and safety, and cost-effectiveness. The WHO model lists guide the development and updating of national essential medicines lists, in accordance with local priorities and treatment guidelines.
“New changes to WHO’s Model List of Essential Medicines will make it easier for TB programmes and technical partners to implement recent updates to WHO guidelines”, said Dr Farai Mavhunga, head of the Unit on Vulnerable Populations, Communities and Comorbidities at the WHO Global Tuberculosis Programme. “It is important for countries and partners to work together to increase access to quality-assured, affordable medicines for people of all ages with TB everywhere”.