Situation at a glance
On 7 June 2023, Brazil notified the World Health Organization (WHO) of a fatal laboratory-confirmed human case of infection with a swine-origin influenza A(H1N1) variant (v) virus in the inner state of Paraná.
Sporadic human cases of influenza A(H1N1)v have been reported previously, including from Brazil. According to the International Health Regulations (IHR) 2005, a human infection caused by a novel influenza A virus subtype is an event that has the potential for high public health impact and must be notified to the WHO.
Based on the information currently available, WHO considers this a sporadic case, and there is no evidence of person-to-person transmission of this event. The likelihood of community-level spread among humans and/or international disease spread through humans is low.
Description of the case
On 7 June 2023, the Brazil IHR National Focal Point (NFP) notified WHO of a fatal human infection caused by a swine-origin influenza A(H1N1)v virus detected by the National Influenza Centre (NIC), Oswaldo Cruz Foundation, Rio de Janeiro.
The patient was a 42-year-old woman with underlying medical conditions who lived near a swine farm. She developed fever, headache, sore throat, and abdominal pain on 1 May 2023 and was hospitalized on 3 May with a severe acute respiratory infection. On 4 May, the patient was admitted to the Intensive Care Unit (ICU) and she passed away on 5 May.
Ongoing investigations reported that the patient did not have any direct contact with pigs, however, two of her close contacts worked at the swine farm. The two contacts did not develop respiratory disease and tested negative for influenza. To date, no human-to-human transmission associated with this case has been identified.
During hospitalization, a nasopharyngeal swab sample was collected from the patient for influenza and SARS-CoV-2 testing, as part of regular respiratory virus surveillance activities. Real-time Polymerase Chain Reaction (RT-PCR) was conducted at the State of Paraná Central Public Health Laboratory, where the sample was subtyped as an influenza A/H1 virus. The sample also tested positive for a swine influenza A virus marker by RT-PCR.
The specimen was forwarded to the National Influenza Centre Oswaldo Cruz Foundation, in Rio de Janeiro, where further complementary analyses and genomic sequencing were performed. Samples received at the NIC on 25 May were confirmed to be an influenza A(H1N1)v virus by sequence analysis on 30 May. The recovered genome has a high identity (99%) with the haemagglutinin (HA) of other Influenza A(H1N1)v viruses previously detected in the municipality of Toledo state of Paraná in 2022. In addition, it has 96% identity with the HA of viruses collected from pigs in Brazil in 2015.
On 8 June, after the Brazilian Ministry of Health (MoH) notified WHO under the IHR, the NIC started the process to send the patient’s samples to the WHO Collaborating Centre at the United States Centers for Disease Control and Prevention (US CDC) for further characterization.
Epidemiology of the disease
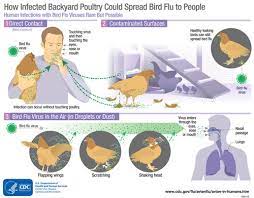
Influenza A(H1) viruses are enzootic in swine populations in most regions of the world. When an influenza virus that normally circulates in swine is detected in a person, it is called a “variant influenza virus”. H1N1, H1N2 and H3N2 are major subtypes of swine influenza A viruses in pigs and occasionally infect humans, usually after direct or indirect exposure to pigs or contaminated environments.
Human infections with variant viruses tend to result in mild clinical illness, although some cases have been hospitalized with more severe disease and some have been fatal.
To date, sporadic human infections caused by influenza A(H1N1)v and A(H1N2)v viruses have been reported in Brazil, and there has been no evidence of sustained human-to-human transmission.
This is the first human infection caused by an influenza A(H1N1)v virus reported in 2023 in Brazil, and the third human infection reported in the state of Parana (the first was detected in 2021 and the second in 2022).
Public health response
Local and national health authorities implemented the following public health measures:
- Conducting further epidemiological investigations, and follow-up of contacts in the family, community, and health care facilities.
- Monitoring the surveillance of influenza-like illness (ILI) and severe acute respiratory infections (SARI) in the surrounding municipalities (within the same health region), particularly influenza virus, seeking to analyze the behavior and trends of respiratory viruses in the region.
- Reinforcing the vaccination campaign for seasonal influenza in at-risk groups.
WHO risk assessment
This is a sporadic case based on currently available information and further spread has not been detected.
Limited, non-sustained human-to-human transmission of variant influenza viruses has been described, although ongoing community transmission has not been identified. Current evidence suggests that these viruses have not acquired the ability of sustained transmission among humans.
There is no vaccine for Influenza A(H1N1)v infection currently licensed for use in humans. Seasonal influenza vaccines against human influenza viruses are generally not expected to protect people from influenza viruses that normally circulate in pigs, but they can reduce the likelihood of getting sick with both human and variant influenza viruses.
WHO assesses the risk of international disease spread through humans and/or community-level spread among humans posed by this event as low. The risk level will be amended if warranted by the investigations currently being conducted by national authorities.
WHO advice
Surveillance:
- This case does not change the current WHO recommendations on public health measures and surveillance of seasonal influenza.
- Due to the constantly evolving nature of influenza viruses, WHO continues to stress the importance of global surveillance to detect virological, epidemiological and clinical changes associated with circulating influenza viruses that may affect human (or animal) health and timely virus sharing for risk assessment.
- Continued vigilance is needed within affected and neighboring areas to detect infections in animals and humans. Collaboration between the animal and human health sectors is essential. As the extent of influenza viruses circulating in animals is unclear, epidemiologic and virologic surveillance and the follow-up of suspected human cases should continue systematically. Guidance on the investigation of non-seasonal influenza and other emerging acute respiratory diseases is available on the WHO website.
- Vigilance for the emergence of novel influenza viruses of pandemic potential should be maintained. WHO has developed practical guidance for integrated surveillance in the context of the cocirculation of SARS-CoV-2 and influenza viruses.
- It is critical that influenza viruses from animals or from people are fully characterized in appropriate animal or human health influenza reference laboratories. Under WHO’s Pandemic Influenza Preparedness (PIP) Framework, Member States are expected to share influenza viruses with pandemic potential on a regular and timely basis with the Global Influenza Surveillance and Response System (GISRS).
Notification and investigation:
- All human infections caused by a novel influenza subtype are notifiable under the IHR, and State Parties to the IHR are required to immediately notify WHO of any laboratory-confirmed case of a recent human infection caused by an influenza A virus with the potential to cause a pandemic. Evidence of illness is not required for this report.
- In the case of a confirmed or suspected human infection caused by a novel influenza virus with pandemic potential, including a variant virus, a thorough epidemiologic investigation of history of exposure to animals, of travel, and contact tracing should be conducted. The epidemiologic investigation should include early identification of unusual respiratory events that could indicate person-to-person transmission of the novel virus. Clinical samples collected from the time and place that the case occurred should be tested and sent to a WHO Collaboration Centre for further characterization.
Travel and trade:
- WHO does not recommend any travel and/or trade restrictions for Brazil based on available information on this event.
Prevention measures for travelers:
- WHO advises that travelers to countries with known outbreaks of animal influenza should avoid farms, contact with animals in live animal markets, entering areas where animals may be slaughtered, or contact with any surfaces that appear to be contaminated with animal excreta. Travelers should also wash their hands often with soap and water. All individuals should follow good food safety and hygiene practices.
- WHO does not advise special traveler screening at points of entry or restrictions with regard to the current situation of influenza viruses at the human-animal interface.
Further information
- PAHO/WHO. Influenza at the Human-Animal Interface: PAHO Recommendations to Strengthen Intersectoral Work for Surveillance, Early Detection, and Investigation, 9 July 2020
- PAHO/WHO. Strengthening the intersectoral work for Influenza at the Human Animal Interface in the Region of the Americas: Technical Questions and Answers. May 2023
- WHO. Global Influenza Programme
- WHO. Influenza virus infections in humans. October 2018
- Case definitions for diseases requiring notification under the IHR (2005)
- International Health Regulations (2005) – Third edition
- World Health Organization. (2011). Manual for the laboratory diagnosis and virological surveillance of influenza. World Health Organization. https://apps.who.int/iris/handle/10665/44518
- Terms of Reference for National Influenza Centers of the Global Influenza Surveillance and Response System. https://cdn.who.int/media/docs/default-source/influenza/national-influenza-centers-files/nic_tor_en.pdf?sfvrsn=93513e78_30
- World Health Organization. (2018). Protocol to investigate non-seasonal influenza and other emerging acute respiratory diseases. World Health Organization. https://apps.who.int/iris/handle/10665/275657. License: CC BY-NC-SA 3.0 IGO
- World Organization for Health Animal (OIE). Swine influenza
Citable reference: World Health Organization (16 June 2023). Disease Outbreak News; Influenza A(H1N1) variant virus – Brazil. Available at: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON473