Falsified DYSPORT (Clostridium botulinum type A toxin-haemagglutinin complex) identified in the WHO Regions of Europe and Eastern Mediterranean
Alert Summary
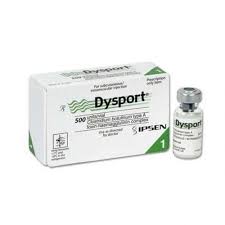
This WHO Medical Product Alert refers to five falsified batches of DYSPORT detected in five countries – Jordan (May 2022), Türkiye (May 2022), Kuwait (June 2022), United Kingdom (June 2022), and Poland (July 2022).
DYSPORT is indicated to treat symptoms of cervical dystonia, glabellar lines (wrinkles), and spasticity.
The genuine manufacturer of DYSPORT is IPSEN.
The genuine manufacturer has confirmed that all of the products referenced in this Alert are falsified on the basis that they deliberately/fraudulently misrepresent their identity and source.
The variable data (batch numbers and manufacturing-expiry dates) are falsified, there are discrepancies in the packaging languages, printing errors on the cartons, and discrepancies in vial types.
Risks
The safety, sterility, and quality of the products referenced in this alert are unknown.
DYSPORT is injected intramuscularly. These falsified products may pose a particular high risk to patients.
It is important to detect and remove these falsified products from circulation to prevent harm to patients.
Advice to regulatory authorities and the public
WHO requests increased surveillance and diligence within the supply chains of countries and regions likely to be affected by these falsified products.
All medical products must be approved and obtained from authorized/licensed suppliers. The products’ authenticity and physical condition should be carefully checked. Seek advice from a healthcare professional when in doubt.
If you are in possession of these falsified products, please do not use them.
If you have used these products, or you suffered an adverse reaction/event after use, you are advised to seek immediate medical advice from a qualified healthcare professional and report the incident to the National Regulatory Authority or National Pharmacovigilance Centre.
National regulatory/health authorities are advised to immediately notify WHO if these falsified products are discovered in their country.
If you have any information concerning the manufacture and/or distribution of these products, please inform WHO via [email protected]
Please click here for the list of falsified products